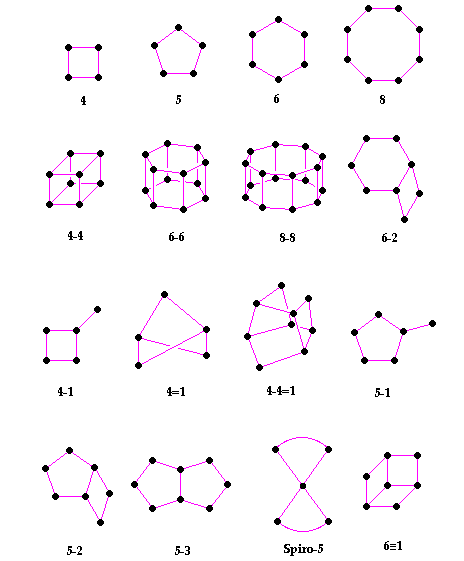
 |
| Secondary Building Units (SBU's) in zeolites. The corner of the polyhedra represent tetrahedral atoms |
 |
| Secondary Building Units (SBU's) in zeolites. The corner of the polyhedra represent tetrahedral atoms |
In the above table - the T atom of the TO4 tetrahedron is located at each of the corners, and the oxygens are located towards the mid-points of the lines joining each T atom (the oxygens are not shown to aid clarity).
These secondary building units (SBU's) - (the primary building units being the TO4 tetrahedra) - can contain up to 16 T atoms. It can be noted that SBU's are non-chiral (neither left or right "handed"). A unit cell always contains the same number of SBU's, and although rare, some materials can have different combinations of SBU's within the zeolite framework.
The framework may be considered in terms of large polyhedral building blocks
forming characteristic cages. For example, sodalite, zeolite A and zeolite X/Y (Faujasite) can all be generated by the truncated octahedron known as the b-cage - see below:
| SODALITE | ||||
 | ||||
| 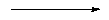
| ZEOLITE A | ||
| Sodalite or b-cage |
 | |||
| ZEOLITE X/Y |
The b-cage has both four-membered and six-membered rings in its structure. The cage formed has an internal diameter of approx. 6Å - sufficient to encapsulate small molecules. By fusing together the four-membered rings, sodalite is formed - and is a naturally occuring material. By bridging (not fusing) the four-membered rings, zeolite A is formed. This does not occur in nature, but is industrially produced on a massive scale for its use in ion exchange, gas separation and drying. If the six-membered rings are bridged, then possibly the most important zeolite structure, zeolite X/Y is formed. The X/Y refers to the silicon/aluminum ratio. This structure has very large microporous spaces, allowing organic molecules to diffuse in and out. This zeolite is one of the most important catalytic systems, and is used in the cracking of long chain hydrocarbons into shorter chain length fuels.
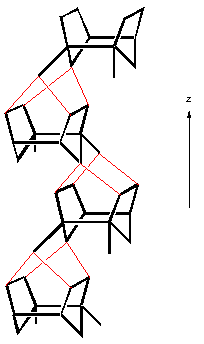
Another important class of materials are the pentasil zeolites, named so because they are constructed of five-membered rings. The most important example is that of ZSM-5 - which is shown below:
 |
| Figure a) |
 |
| Figure b) |
 |
| Figure c) |
| Figure d) |
| a) SBU in pentasil zeolites, b) SBU linked chains, c) Layer formed from linked chains in ZSM-5 structure, and d) Chimed model of ZSM-5. |
ZSM-5 is used in a variety of catalytic applications, including the catalysis of methanol into fuel hydrocarbons. The individual pentasil units are shown in Fig. a) above. These combine to form long chains, Fig b), which then join together to form layers, Fig. c).
One interseting problem in zeolite chemistry is the distribution of silicon and aluminium atoms among the T sites. According to Lowensteins' rule, Al-O-Al linkages in zeolitic frameworks are forbidden. As a result, all aluminate tetrahedra must be linked to four silicate tetrahedra, and in general this is proved to be the case, but recent investigations into zeolites synthsised at high temperatures have shown non-Lowenstein distributions in sodalite materials.
The range of Si/Al ratios varies between zeolites. ZSM-5 is a high silicate zeolite, whereas zeolite X/Y can be prepared in high silicate forms, or high aluminate forms, but is usually produced with a Si/Al ratio close to unity with a fully ordered Si-Al distribution over the tetrahedral sites, in accordance with Lowenstein's rule.
The inclusion of aluminium into the zeolite structure has two major effects:
Many other atoms can be substituted into zeolite frameworks, eg. gallium to form gallo silicates, but transition metals such as iron, cobalt and titanium have also been introduced and has a great effect on the catalytic properties of each zeolite.
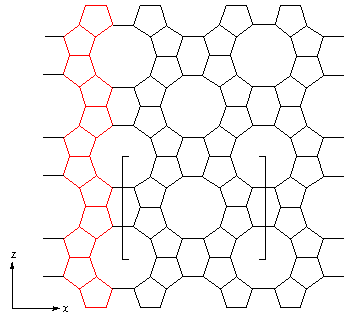
Zeolite silicates and phosphates apparently constitute two distinctive categories of matrerials, but there are in fact three, as shown in the table below, the third category comprising of zeolites with structures common to both silicates and phosphates. [For more information on each type - please click on Atlas.]:
| Silicates | Both Silicates and Phosphates | Phosphates |
 |  |  |