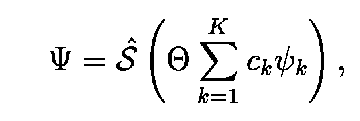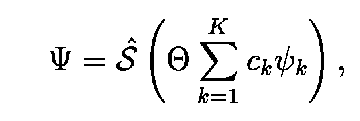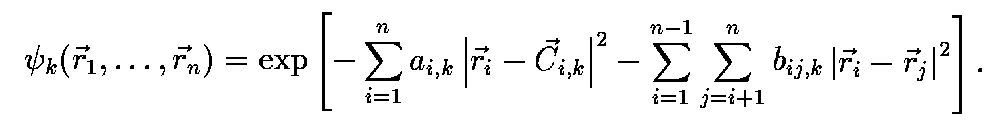Explicitly Correlated Gaussian Functions in Variational
Calculations.
Four electron atomic and molecular systems.
Jacek Rychlewski and Jacek Komasa
A. Mickiewicz University, Department of Chemistry,
Grunwaldzka 6, 60-780 Poznan, Poland
and
Poznan Supercomputing and Networking Center,
Wieniawskiego 17/19, Poznan, Poland
Exponentially correlated Gaussian functions (ECG) were introduced into
quantum
chemistry by Boys and Singer in 1960. Since then they have been used
with varying success but generally considered as inferior to
wave functions with linear correlation factors.
A full definition of the ECG wave function is as follows:
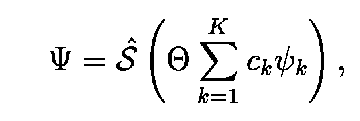 S - an operator ensuring proper electron and space
symmetry of the function.
Theta is properly chosen spin function.
The space part of the basis function is defined as
S - an operator ensuring proper electron and space
symmetry of the function.
Theta is properly chosen spin function.
The space part of the basis function is defined as
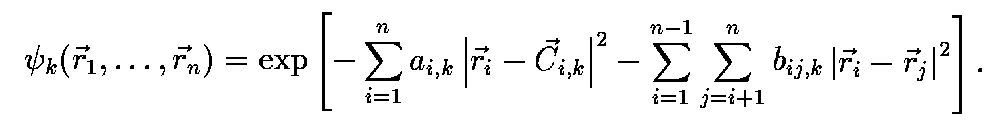 where i,j run over all the n electrons,
and a_{i,k}, b_{ij,k}, C_{i,k} are nonlinear parameters
determined variationally.
As we have discovered, careful
optimization of the nonlinear variational parameters
enables to obtain much better results
than previously expected. We have used a modified Powell's conjugate
directions approach and updating algorithms for solving secular
equations. Our method was applied so far to the following systems:
ground states of LiH, H_3 [1], He_2^+ [2], H_2 [3],
HeH^+ [4], beryllium and lithium atoms, helium dimer He_2, selected
excited states of H_2 and the potential energy surface of H_3^+. In
all these cases we have obtained significantly lower energies than
previously published variational results, including those produced by
the Kolos-Wolniewicz wave function for H_2 and HeH^+.
In the communication the energies and selected properties of four
electron atomic and molecular systems: Be, He_2 and LiH, will be
presented.
It will be demonstrated that
exponentially correlated Gaussians represent today the best basis for
accurate variational calculations of four-electron atoms and molecules.
where i,j run over all the n electrons,
and a_{i,k}, b_{ij,k}, C_{i,k} are nonlinear parameters
determined variationally.
As we have discovered, careful
optimization of the nonlinear variational parameters
enables to obtain much better results
than previously expected. We have used a modified Powell's conjugate
directions approach and updating algorithms for solving secular
equations. Our method was applied so far to the following systems:
ground states of LiH, H_3 [1], He_2^+ [2], H_2 [3],
HeH^+ [4], beryllium and lithium atoms, helium dimer He_2, selected
excited states of H_2 and the potential energy surface of H_3^+. In
all these cases we have obtained significantly lower energies than
previously published variational results, including those produced by
the Kolos-Wolniewicz wave function for H_2 and HeH^+.
In the communication the energies and selected properties of four
electron atomic and molecular systems: Be, He_2 and LiH, will be
presented.
It will be demonstrated that
exponentially correlated Gaussians represent today the best basis for
accurate variational calculations of four-electron atoms and molecules.
Citations
- W. Cencek and J. Rychlewski, J. Chem. Phys. 98, 1252 (1993).
- W. Cencek and J. Rychlewski, J. Chem. Phys. 102, 2533 (1995).
- J. Rychlewski, W. Cencek, and J. Komasa,
Chem. Phys. Lett. 229, 657 (1994).
- J. Rychlewski, Int. J. Quant. Chem. 49, 477 (1994).
- J. Komasa, W. Cencek, and J. Rychlewski,
Phys. Rev. A, 52, 4500 (1995).
- J. Komasa and J. Rychlewski, Chem. Phys. Lett., 249, 253 (1996).
This work was supported by KBN grant T11F 010 08p01.