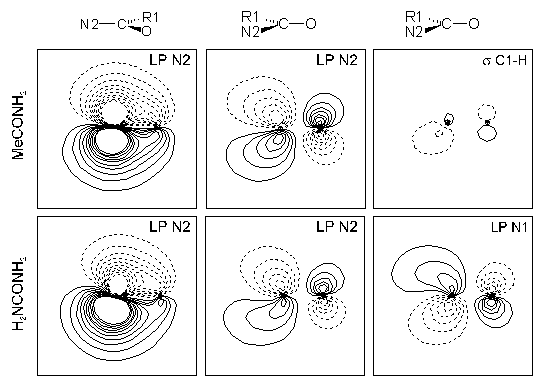
Electron distributions of 29 acyl compounds are characterized to be influenced by concurrent resonance at the carbonyl group (see fig. 1). Substituent effects on molecular properties like geometries, net atomic charges and conformational flexibilities can be related to this description [4]. Effects of solvation with explicit water molecules and with an electrostatic polarized continuum on electron densities and related properties are shown [5].

Fig. 1: Selected Natural Localized Molecular Orbitals of acetamide and urea. Both molecules are not planar in the gas phase, but show a higher amount of resonance stabilization at the CO group than formamide.
Basicities of 15 carboxylic acid derivatives and ureas at the carbonyl oxygen and at the leaving group are calculated in the gas phase (selected structures see fig. 2). Effects of protonation on charge densities including the resonance description are discussed. Calculated pKa values of single protonated acyl compounds in aqueous solution (see table 1) based on relative basicities from SCI-PCM-calculations and an extrapolation function developed from 10 aliphatic, alicyclic and aromatic nitrogen bases can be used to derive hypotheses about initial steps of nucleophilic substitution reactions in acidic solutions.
| Molecule | pKa (C=O) | pKa (-X-) | expt. [6] |
| Formaldehyde | -12.2 | - | - |
| Acetaldehyde | -8.7 | - | - |
| Acetone | -6.5 | - | -7.2 |
| Methylformate | -8.8 | -17.5 | - |
| Methylacetate | -6.9 | -15.7 | -7.2 |
| Phenylformate | -10.3 | -18.4 | - |
| Methylbenzoate | -7.2 | -16.4 | -7.3 |
| Formamide | -2.6 | -8.3 | -2.0 |
| Acetamide | -0.9 | -4.8 | -1.3 |
| Methylformamide | -1.2 | -6.9 | -1.6 |
| Methylacetamide | 0.4 | -4.4 | -0.5 |
| Urea | 2.3 | -0.6 | 2.0 |
| Methylurea | 2.0 | 0.0 / -0.2 | - |
| N,N'-Dimethylurea | 1.8 | 1.2 | - |
| N,N-Dimethylurea | 2.0 | -0.8 / 0.0 | - |
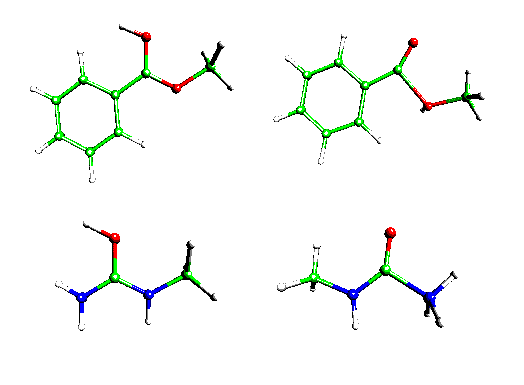
Fig. 2: Structures of protonated methylbenzoate and methylurea. Protonation of the carbonyl oxygen yields additional resonance stabilization at the CO group. Protonation of the leaving group yields pre-fragmentation of the molecules.
Possible pathways for the reaction of a simple ester, amide and urea with a water molecule in neutral and acidic aqueous solution are characterized by stationary points on adiabatic potential hypersurfaces and by intrinsic reaction coordinates. The systems include two additional explicit water molecules, which can act as bifunctional acid-base catalysts (see fig. 3). Related models for formamide were published in [7]. The reaction models allow hypotheses about preferred reaction pathways for hydrolyses of different carboxylic acid derivatives and about the role of catalytic solvent molecules. Sources of different reactivities against a nucleophile are discussed in terms of resonance at the reaction centre and influences of protonation on it.
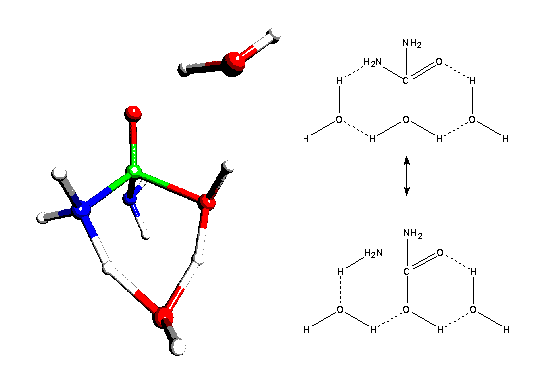
Fig. 3: Transition state for the neutral water assisted hydrolysis of urea through a concerted one-step mechanism (Becke3LYP/6-31G*). An addition-elimination mechanism is found to be less preferred for this substrate.
The study was granted by the Deutsche Forschungsgemeinschaft DFG-Gz. INK16/A1-1.