
Laurence P. Cuffe, Noel J. Fitzpatrick
Department of Chemistry, University College Dublin , Belfield, Dublin 4, Ireland.
HMPX (M = Li, Na X = S, O) molecules were examined using ab initio MO methods. All species derived from HMPX by abstracting one or two atoms were considered. Geometries were determined at the MP2-FC/6-311++G** level with relative energies estimated by single point calculations at the QCISD(T)/6-311++G** level including zero point corrections. The structures, relative stabilities and heats of formation of the most stable species are discussed. Harmonic vibrational frequencies have been obtained at both the HF/6-311++G** and MP2/6-311++G** levels and these are presented. For the heaviest molecule under investigation (HNaPS) results were calculated at the MP2-FU/6-311++G** level. The results show that the addition of lithium or sodium to HOP and HPO reduces the energy difference between the species, without changing the relative energy ordering. Similarly, the addition of lithium or sodium to HPS and HSP also reduces the energy difference between the species, without changing the relative energy ordering.
In this work we examined the effect of the alkali metals Lithium and Sodium on the structure and relative stability of the molecules HPO, HOP, HPS, HSP. The aim of this work was to further our understanding of the electron transfer process from alkali metals in small systems and their effect on stability and reactivity.
Each of the four triatomic molecules above has been examined previously. For both HPO and the HSP one isomer is significantly lower in energy (more stable) than the other. We wished to examine the possible effect of Li and Na on the structure and relative stability of these isomers. HOP is also important as a molecule implicated in the interstellar role of phosphorous1 . The fluorescence of HOP is2 also used in the analytical determination of phosphorous and the possible effect of Li, Na on this could also be of interest.
We first examined both conformers for each of the above molecules and found a transition state connecting HPO to HOP. We then examined all four of the above molecules using a detailed basis set 6-311++G** and an MP2-FC correlated method to optimise their structures. Geometries were determined at the MP2-FC/6-311++G** level. Relative energies were estimated by combining single point calculations at the QCISD(T)/6-311++G** level with a zero point correction derived from calculations at the MP2-FC level. These states were further characterised by frequency analysis at both the HF and MP2 levels using the same basis set.
We then carried out a search for possible stationary points for each of the species HLiOP, HNaOP, HLiSP, HNaSP. This was also carried out using the 6-311++G** basis set and both HF and MP2 levels of theory. Once more the states found were characterised using frequency calculations at both the HF and MP2-FC level.
As the HNaSP species contained the heaviest atoms in this study, it was chosen for this investigation. To examine the effect of using a frozen core instead of a fully correlated set of orbitals we re-optimised all states found for the HNaSP molecule using MP2-FU and QCISD(T)-FU for the single point energies. We found no significant differences.
Structures for HPO, HOP, HSP and HPS are first presented. Energies are presented in kcal/Mole. The absolute energy of the most stable species is given in the caption. The energies for all species were calculated using
E(Total)= 0.93(MP2 zero point energy) + E(QCISD(T))
To aid comparison the energies of each of the other species are expressed, in kcal/Mol, relative to the most stable species. Absolute energies of all molecules considered are given in Table 1 below.

Structures of HPO,HOP,HSP,HPS.

Structures of H,Li,O,P

Structures of H,Na,O,P
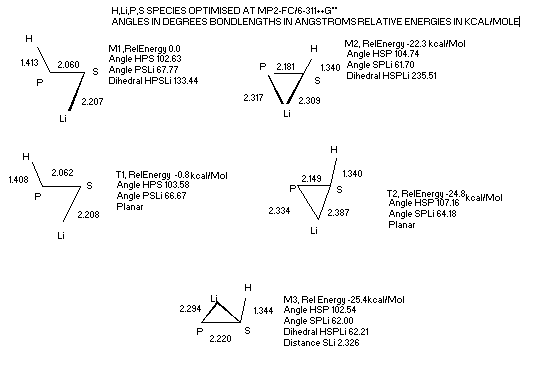
Structures of H,Li,P,S

Structures of H,Na,P,S
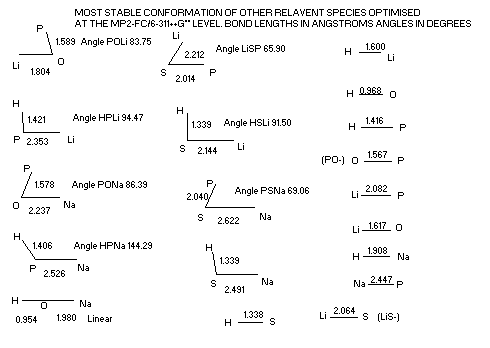
Structures of some other species derived from HMPX (M=Li, Na, X=S, O)
only the most stable conformations of each species is presented.
Table 1. Computed energies of all molecules considered. Table 2 below gives uncorrected MP2 harmonic vibrational frequencies for the most stable conformations of the species presented above.
Table 2 MP2 Frequencies for selected species
The addition of Li to the HOP HPO system reduces the energy difference between isomers by 12.76kcal/Mol.
The addition of Na to the HOP HPO system reduces the energy difference between isomers by 15.26 kcal/Mol.
The addition of Li to the HPS HSP system reduces the energy difference between isomers by 5.3 kcal/Mol.
The addition of Li to the HIPS HASP system reduces the energy difference between isomers by 6.4 kcal/Mol.
R. P. A. Bettens, H. H. Lee, The Importance of Classes of Neutral-Neutral Reactions in the Production of Complex Interstellar Molecules, The Astrophysical Journal, Vol 443:Pgs 664-674, April 20 1995.
B. E. Turner, Observations and Chemistry of Interstellar Refractory Elements, The Astrophysical Journal, Vol 376:pgs 573-598, August 1 1991.
P. Redondo, A. Largo, J. M. Ugalde, J. Phys. Chem. Vol 95: pgs 4318-4323,1991.
B. E. Turner, T. Tsuji, J. Bally, M. Guelin, J. Cernicharo, Phosphorus in the Dense Interstellar Medium, The Astrophysical Journla Vol 365: pgs 569-585, December 20 1990.
L. L. Lohr. R. C. Boehm, Ab Initio Study of the Gaseous Oxyacids of Phosphorus, Their Conjugate Bases, and Their Corresponding Neutral Radicals, J. Phys Chem Vol 91: pgs 3203-3207, 1987.
M. T. Nguyen, Stuructures and Energies in the Simplest Compounds With P = S Bond: Thioxophosphane (HPS, HPS+) Thiohydroxyphosphinidene ( HSP and HSP+). Chem Phys. Vol 117(1),pgs 91-99, 1987.
J. A. Boatz, M. W. Schridt, M. S. Gordon, Ground-State Potential Energy Surface of Phosphine Oxide, J. Phys. Chem.,Vol 91:1743-1749, 1987.
P. A. Hamilton, T. P. Murrells, Mechanism for the Chemlluminescence in Oxygen-Phosphorus Systems, J. Phys Chem. Vol 90: pgs 182-185, 1986.
M. T. Nguyen, A. F. Hegarty, P. Brint, An Ab Initio Study of the Ground and Excited States of HPO, Chem. Phys. Vol 98 Pgs 447-453, 1985.
L. L. Lohr, A theoretical Study of the Gaseous Oxides PO2 and PO, Their Anions, and Their Role In the Combustion of Phosphorus and Phosphine, J. Phys. Chem, Vol 88: pgs 5569-5574.1984.