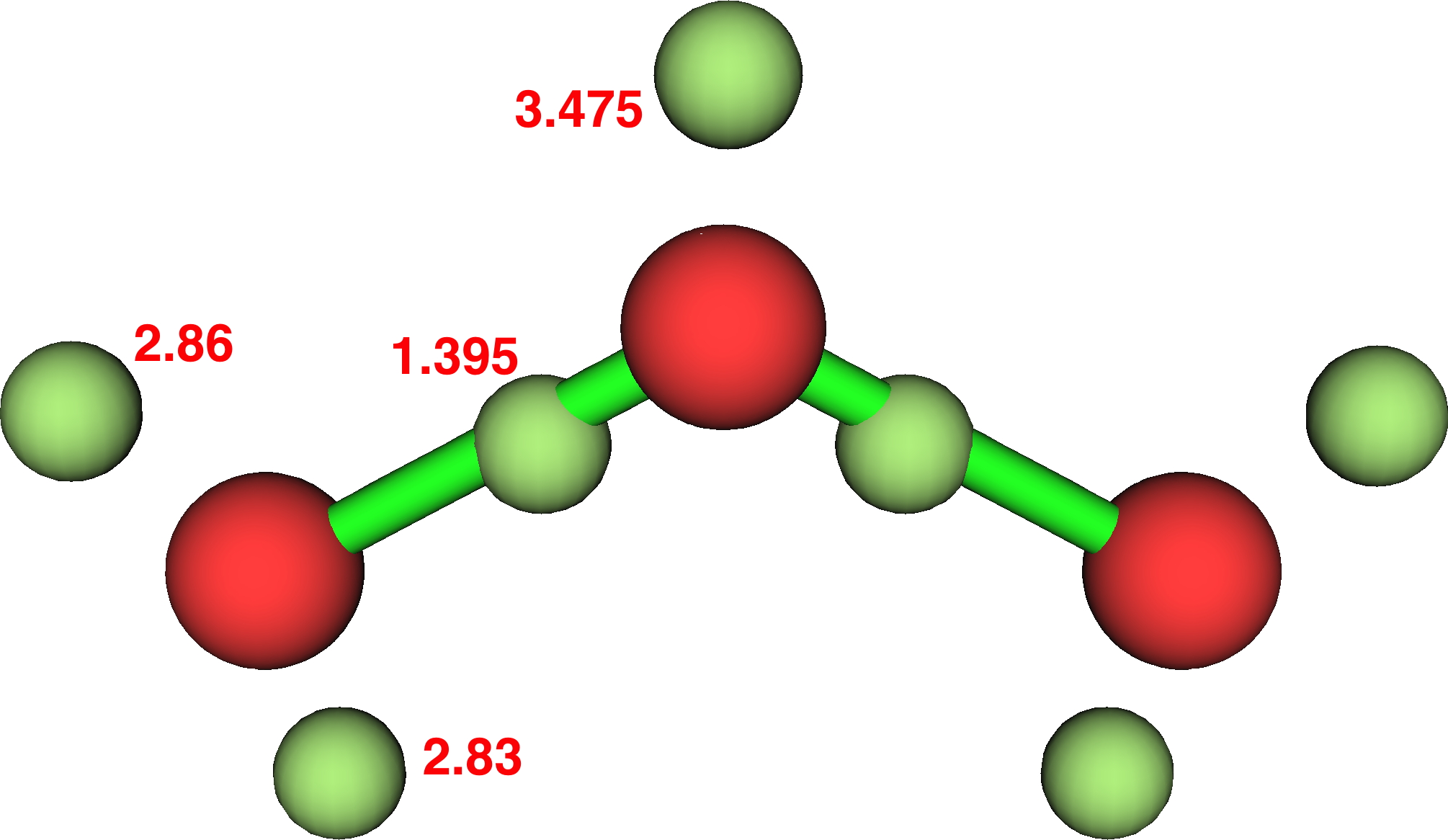
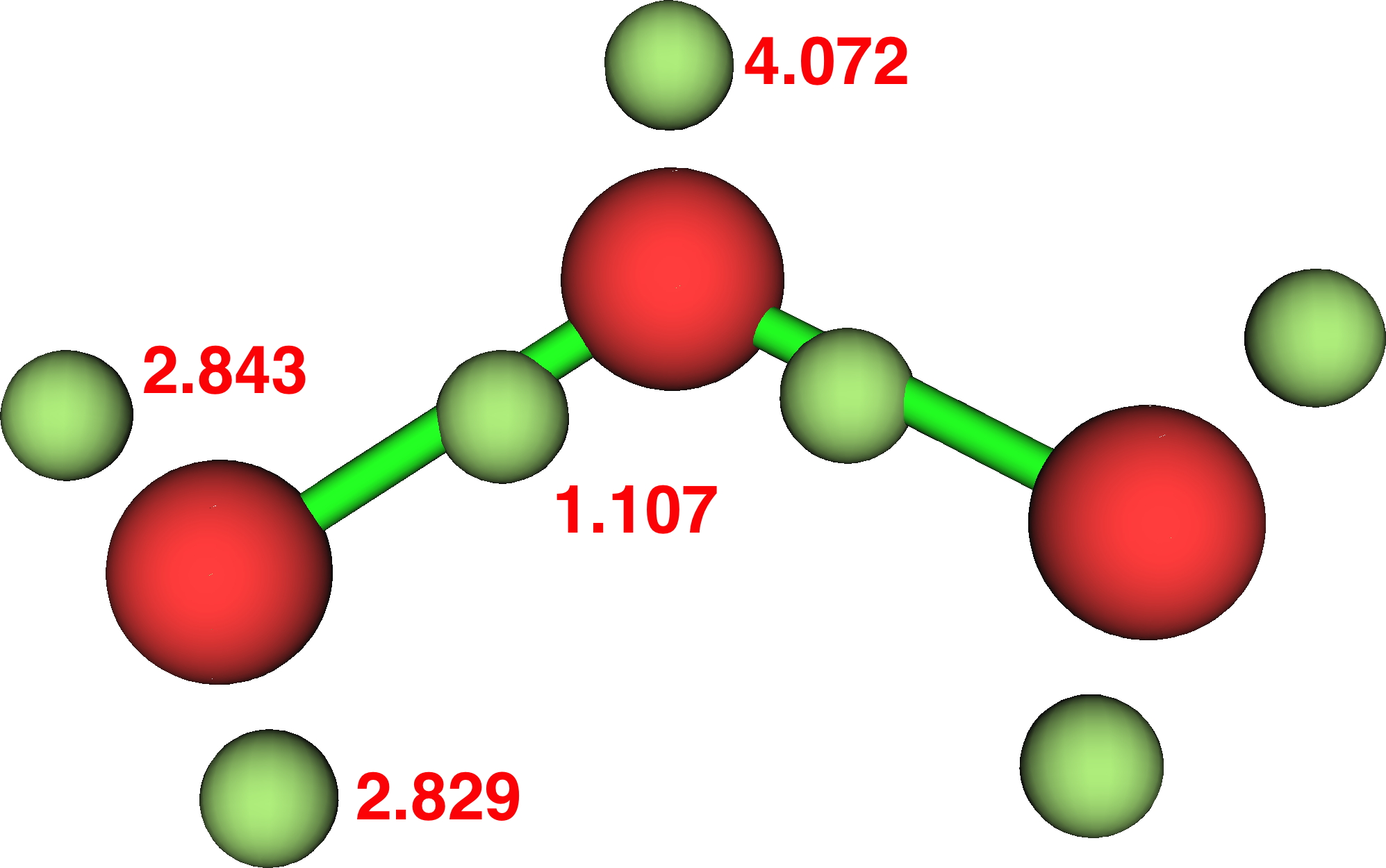
Ozone (Durant, 2015, doi: chpz):
-
Valence electron equivalent γ: the formal
shared electron count at a given atom, obtained by any combination of valid ionic and
covalent resonance forms that reproduces the observed charge distribution.
If γ(X) > 8, neither form of the octet rule is obeyed and the atom is
hypervalent.
- Thus ozone: γ(O) =9.52 (hypervalent, table 2!)
- NBO Bond indices: C, 3.716; N 3.802; N 2.907; N=N order= 2.368
- ELF: O, 7.09, 6.27, 7.09 (DFT), 6.78, 6.29, 6.78 (CAS)
FAIR Data: 10.14469/hpc/3476. See also
diazomethane.