There are many different types of alcohols, which is the name given to organic molecules containing the -OH group. The lower alcohols are liquids with characteristic odours and sharp tastes. They have a relatively high boiling point due to Hydrogen bonding. However, the one we are most familiar with is ethanol, (C2H5OH) which is the one derived from the 2 carbon molecule, ethane.
![]() Ethanol (ethyl alcohol)
Ethanol (ethyl alcohol)
Dilute solutions of ethanol in the form of beer or wine have been known since the dawn of civilization, and it is likely that ethanol was the first substance to be synthesised by humans. It has even been suggested that the discovery of alcohol fermentation (and the physiological effects it produces) provided a major incentive for agriculture and the start of civilization!
 A wine cellar
A wine cellar
Ethanol was originally produced by mixing honey, fruits, berries, cereals or other plant materials and simply leaving them in the sun. This created a liquid which was prized as a food, used as a potion in ceremonial or religious rites, and administered as a medicine. The first known brewery dates from about 3700 B.C. in Egypt. It is believed that wine was discovered later in ancient Mesopotamia. The story goes that a young concubine of the King had fallen out of favour, and rather than face his rejection, she decided to commit suicide. So she went to the cellars where the grapes were stored (grapes were a valuable source of storable sugar at the time). This was potentially hazardous, since yeast on the skins of the grapes would often begin fermenting with any juice that had escaped from the fruit, and the CO2 that was produced could build up in the closed cellars to lethal levels. So, while sitting there waiting to die from CO2 asphyxiation, she decided to speed the process up by drinking some of the 'poisonous' juice that had formed among the grapes. After drinking rather a lot of this strange-tasting juice, she became so drunk that she forgot why she'd gone to the cellar and ran back to the King. The King was fascinated by her abrupt change in behaviour, and, on hearing about the juice in the cellar that had caused this transformation, rapidly went there to try some for himself...
Most wines contain less than about 13% ethanol by volume, since the yeast that performs the fermentation process cannot survive in higher concentrations. Distilling the beers or wines can increase the ethanol content up to about 35-50%, which is typical of most whiskey or brandy. The alcohol content, however, is often expressed as proof. This is simply twice the percentage alcohol by volume, so that a 100 proof whisky would contain 50% ethanol. The term 'proof' was first used in the 17th century by whiskey dealers. Unscrupulous dealers used to add water to their whiskey to increase their profits, so to combat this, whiskey was tested by pouring a sample onto some gunpowder and setting it alight. If the gunpowder ignited after the alcohol had burned off, it was proof that the whiskey had not contained too much water.
Ethanol occupies a unique position, it is both an important industrial chemical and a highly taxed beverage. Therefore it must be made available to the chemical industry in a form that cannot be drunk. Therefore, a denaturant is added, which is a substance which makes the mixture both unpalatable and poisonous. There are over 80 legal denaturants, but the most common are methanol and high-test gasoline. Methylated spirits is an example of a familiar denatured alcohol. This is mainly ethanol which contains a small proportion of methanol, and is commonly used as a household solvent, for example, in cleaning paintbrushes.
Since ethanol is completely soluble in water, when you drink an alcoholic beverage, the ethanol molecules are rapidly absorbed through the stomach and small intestines and pass into the bloodstream. Once there, they moves rapidly into the tissues, especially into organs with large blood supplies such as the brain. This continues until the concentration of ethanol in the tissues and that in the blood are equal. Because the ethanol becomes equally distributed throughout all the tissues in the body, the concentration of ethanol in the breath or urine is a pretty accurate indicator of the level in the blood, which is why a breathalyser test is used to determine the blood alcohol level of a driver.
 A police officer conducting a breath test
A police officer conducting a breath test
The ethanol molecule is actually poisonous, but so long as the amount taken in one go is not too large, or it is not used too frequently, it is easily metabolised and poses no health problem. Small amounts of alcohol act as a stimulant to many organs, but with increasing levels it begins to act as a depressant. This is especially true in the brain, where ethanol disrupts the nerve cell membranes making the brain less receptive to stimuli. With a blood alcohol level of less than 0.1% a person usually becomes pleasantly relaxed, with an easing of tension and anxieties. Also, as the control centre in the brain becomes less active, inhibitions become decreased. This is why alcohol is widely used in many cultures as an aid to social events. At higher concentrations, the increased disruption to the nervous system results in a deterioration of muscular coordination, slurred speech and difficulty in comprehension. Pain is also dulled, which is why, before the invention of modern anesthetics, patients were often given large amounts of alcohol to deaden the pain before an operation. Blood alcohol concentrations above 0.36% can result in delirium, coma and even death.
Ethanol is metabolised in the liver, and produces mainly acetaldehyde. This is one of the chemicals mainly responsible for the symptoms of a hangover, although there are a number of other physiological after-effects that occur after imbibing large quantities of the poisonous ethanol molecule that also contribute to the hangover. The final product is acetic acid, which is either oxidised to provide energy, or used by molecules such as coenzyme A to create fatty acids, steroids, and other useful biological molecules. Longer chained alcohols, and related chemicals such as ketones and aldehydes, such as those that are frequently found in wines and some distilled spirits, are metabolised in a similar way, but at a slower rate. This is why some wines and spirits can often produce worse hangovers than purer alcoholic drinks, such as vodka, which largely consists of only ethanol and water.
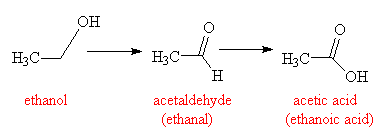
Ethanol is metabolised first to ethanal, then to ethanoic acid.
Methanol (CH3OH) is the alcohol derived from methane, and is the first and simplest of the alcohols. Its common name, wood alcohol, comes from the fact that it was once obtained by heating hardwoods in the absence of air. The word methanol comes from a combination of the Greek words methy ('intoxicate' or 'wine'), and hule ('substance', 'timber', or 'group of trees'). Methanol also occurs in wood smoke, and in small quantities in new wine and some ciders. It is very poisonous, less than 2 teaspoons (2 ml) can cause blindness, and 2 table spoons (30 ml) can cause death. This toxicity is mainly due to it being converted in the body to formic acid and formaldehyde, which first attack the cells in the retina, then the other vital organs.
 |
Methanol is added in small amounts to ethanol in order to make it undrinkable. In this form it is known as methylated spirits, and is used for cleaning paintbrushes. |
Longer chained alcohols also exist, and as the number of carbons increases the complexity also increases, as does the number of structural isomers. The first member of the series after ethanol is propanol (C3H7OH), and this can have two different isomers. The first has the -OH group attached at the end of the chain, and is called propan-1-ol, since the -OH group is attached to the first carbon. The second isomer has the -OH group attached to the middle carbon, and is called propan-2-ol. Its older name was isopropyl alcohol, or IPA, and is used as a common solvent and cleaning agent in chemistry laboratories. Also, because it evaporates rapidly, IPA is widely used in astringents to cool the skin and constrict surface blood vessels.
 | 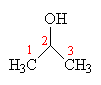 |
| Propan-1-ol | Propan-2-ol (Isopropyl alcohol) |
The next member of the alcohol series is butanol, with 4 carbons. There are many possible isomers of butanol, particularly as the carbons no longer have to arrange themselves in a straight chain. One interesting example of butanol is the butan-2-ol isomer, since this is chiral, that is, it can exist in two identical, but mirror-image forms. The form that rotates plane polarised light to the left is called the S-isomer (S means sinister, which is latin for left), and the one that rotates the plane of the light to the right is called the R-isomer (R is Latin for rectus, or right).
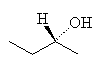 | 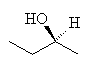 |
| R-butan-2-ol | S-butan-2-ol |
An alcohol molecule can contain more than one -OH group. As the number of hydroxyl groups in the molecule increases, the Hydrogen bonding effect also increases, raising the boiling point and making the liquid more viscous.

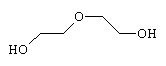
| 
| 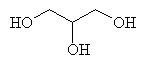 |
| Ethylene Glycol (1,2-ethane diol) | Diethylene Glycol | Glycerol |
Ethylene glycol (1,2-ethane diol) is colourless and water soluble, but just as toxic as methanol when imbibed. It is of great commercial value as antifreeze for cars since it interferes with the intermolecular bonding between water molecules and prevents them from solidifying until the temperature has been reduced to much lower than normal. Also, since it has a reactive -OH group at each end, it can be reacted with carboxylic acids to produce polymeric esters, or polyesters, which are used for artificial fibres. A related material, diethylene glycol (which is also used to some extent as an antifreeze, but is more usually an industrial solvent) caused the notorious Austrian wine scandal of 1985. This happened when the wine was contaminated with this chemical in an attempt to change its taste and viscosity, thereby making it resemble more expensive wines. The wines were quickly withdrawn from sale when traces of the poisonous diol were discovered. Glycerol (or glycerin) has 3 -OH groups, and is very viscous and sweet tasting, and is the molecule the criminals probably should have used since it is often found as a natural ingredient of sweet wines. It is not toxic, and is actually a component of all natural fats and oils (in the form of glycerides).

| Diols are often used as antifreeze in car radiators |