 |  |
| Psilocybe Muscorumi - one of the family of 'magic mushrooms'. | The peyote cactus. Mescaline is made when the caps, or buttons, are dried and eaten, brewed into tea or powdered into capsules. |
Psilocybin is the main active ingredient in so-called 'magic mushrooms'. It has hallucinogenic properties, and is closely related to mescaline in structure. Both chemicals have been known for centuries by the Aztecs in Mexico, who used them in tribal rites, believing the vivid, colourful hallucinations had religious significance. The Aztecs even had professional mystics and prophets who achieved their inspiration by eating the mescaline-containing peyote cactus (Lophophora williamsii). Indeed, the cactus was so important to the Aztecs that they named it teo-nancacyl, or "God's Flesh". This plant was said to have been distributed to the guests at the coronation of Montezuma to make the ceremony seem even more spectacular. During the 19th Century, the use of peyote cacti in tribal rituals spread north to the natives of North America, such as the Comanches, Kiowas and the Mescalero Apaches, from where mescaline obtained its name. Over the years, the use of these drugs in religious rites became fused with Christianity, and even today some tribes believe that God put some of His power into peyote, and Jesus was the man who gave the plant to the Indians in a time of need.
 |  |
| Psilocybe Muscorumi - one of the family of 'magic mushrooms'. | The peyote cactus. Mescaline is made when the caps, or buttons, are dried and eaten, brewed into tea or powdered into capsules. |
Psilocybin itself, comes from 'magic' mushrooms (such as Psilocybe mexicana, Psilocybe muscorumi and Stropharia cubensis), several varieties of which grow in temperate regions of the world, including the USA, Europe and the UK. Their use became fashionable in the UK in the 1970s as a 'natural' and legal alternative to LSD. In most countries it is not illegal to possess or consume these mushrooms, however if they are prepared (e.g. crushed or dried), or deliberately cultivated, they then become a Class A drug punishable by imprisonment.
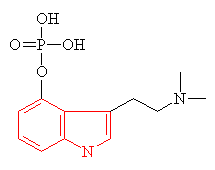 |  |
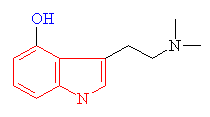
| Psilocybin The indole ring is shown in red | Mescaline The structure resembling an indole ring is shown in red |
Psilocybin and mescaline are psycho-active because they closely resemble the structures of neuro-transmitters that convey impulses from one nerve to another, especially in the brain. Examples are serotonin and norepinephrine. The hallucinogenic molecules fit into the same receptors as the neuro-transmitter, and over-stimulate them, leading to false signals being created.
 |  |
| Serotonin The indole ring is shown in red and the amine group in blue | Psilocin The hydrolysed form of psilocybin with the change shown in blue |
Many hallucinogens are variations of important biological substances called indole-amines. These contain an indole ring structure (highlighted in red in all the diagrams), which is simply a 6-membered benzene ring fused to a 5-membered ring containing nitrogen. An example of an important indole-amine is the serotonin molecule mentioned earlier. The main variation is to add a few methyl groups, making the molecule more lipophilic (soluble in fats), and so better able to penetrate the fatty membranes that protect nerves and nerve endings. This allows the molecules to more readily penetrate the central nervous system, and hence makes them more potent. Psilocybin has this indole ring, with a phosphoric acid group attached to it. In the body the phosphoric acid group is oxidised to the hydroxyl compound, known as psilocin, which is equally psycho-active. Mescaline does not have the indole ring, but as shown in red in the diagram above, its structure can be represented so as to suggest its relation to the ring. The most famous hallucinogenic drug, LSD has a complicated structure, but it, too, is based around the indole ring.
 | Lysergic acid diethylamide - LSD The indole ring is highlighted in red |
The relationship between the hallucinogenic drugs and serotonin has given rise to the hypothesis that schizophrenia is caused by an imbalance in the metabolism of serotonin, with excitement and hallucinations resulting from an excess of serotonin in certain regions of the brain, and depressive and catatonic states resulting from its deficiency.