 | 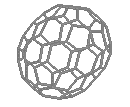 |
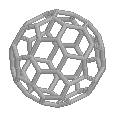
| The spherical C60 | ...and the oval C70 |
For centuries it was believed that the element carbon only existed in 2 very different forms, soft, black, conductive graphite and hard, transparent, insulating diamond. However in 1985 a new form of carbon was discovered, which had a remarkable structure. This form of carbon was composed of hexagons and pentagons of carbon, joined together to form a completely spherical shape - a football!
 |  |
| The spherical C60 | ...and the oval C70 |
The story began back in the early 1970's, when the chemistry of unsaturated carbon-based molecules was being studied by a group at the University of Sussex, led by Harry Kroto and David Walton. They developed methods for synthesising long-chain polyynes (compounds with many triply-bonded carbons), and long-chained polyynylcyanides, HC5N, HC7N and HC9N. These molecules were of interest because they are produced by red giant stars and had been detected by radioastronomy in the cloud material of the interstellar medium.
| A polyyne | |
| A polyynylcyanide |
In the 1980's a technique was developed by Richard Smalley and Bob Curl at Rice University, Texas. They used laser vaporisation of a suitable target to produce clusters of atoms. Kroto realised that if the target were to be made from graphite, the laser apparatus would be ideal to probe the formation of carbon chains, and so planned a collaboration between his group at Sussex and the one at Rice.
The Sussex/Rice experiment took place in September 1985, and the carbon plasma produced by the laser vaporisation was probed by time-of-flight mass spectrometry. The experiments confirmed that large carbon chain/clusters were being formed. But during the experiments it was noted that the peaks for mass 720 (corresponding to 60 carbon atoms) and, to a lesser extent, for 840 (corresponding to 70 carbons), behaved unusually and formed under all conditions as well as exhibiting great stability.
This experimental evidence indicated that a carbon molecule with sixty carbon atoms was forming, but provided little structural information. The research group concluded after reactivity experiments, that the most likely structure was a spheroidal molecule. But it wasn't known exactly how 60 carbons could arrange themselves to form a sphere. But then Kroto recalled seeing the geodesic dome structure at the centrepiece of the Expo '67 exhibition in Montreal, which was a closed hemispherical shape containing linked pentagons as well as hexagons. He realised that this was the way the carbons were arranged in C60, and so the molecule was named after the architect of the geodesic structure, Buckminster Fuller. C60 itself, is therefore known as Buckminster Fullerene, or 'Bucky balls' for short. Other similar closed cage structures, such as the oval C70, were quickly discovered - and the entire family of such cages was called the 'fullerenes'.
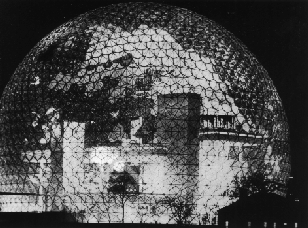
Buckminster Fuller's Dome - Expo '67 Montreal
(Courtesy of B. Eggen, Univ. of Sussex)
For this discovery, the Royal Swedish Academy of Sciences awarded the 1996 Nobel Prize for Chemistry jointly to:
C60 and other fullerenes are now routinely made by a low pressure method in which an electric discharge is passed across the gap between 2 carbon electrodes in a helium atmosphere. The resulting soot is collected and mixed with a solvent such as benzene; the fullerenes dissolve and can be extracted.
Fullerenes are closed cage structures. Each carbon atom is bonded to three others and is sp2 hybridised. Hexagonal rings are present but pentagonal rings are required for the cage to close. The smallest fullerene that has been identified is C20+, and the largest has well over 100 carbons. All fullerenes have an even number of carbons, and if a C2n fullerene is fragmented, by say mass spectrometry, it will spit out C2 fragments and form C2n-2 fullerenes until it gets to C32, at which point the whole structure falls apart.
The most symmetrical, and therefore the most stable, fullerene is C60, followed by C70, with the others being much less stable. The C60 molecule has two bond lengths - the 6:6 ring bonds (between two hexagons) can be considered "double bonds" and are shorter than the 6:5 bonds (between a hexagon and a pentagon). C60 and C70 have similar properties in that they behave very much like electron deficient alkenes and readily react with electron rich species.
Research into the chemistry of these novel molecules is now intense. By reacting the basic fullerene structure in various ways it has been possible to attach chemical groups to the side of fullerenes, place groups or ions inside the spherical structures, link fullerenes together, replace some of the carbon atoms with other atoms such as nitrogen, and many more interesting variations. Recently another remarkable property of the fullerenes has been discovered. If they are reacted with alkali metal ions, such as potassium, the resulting crystals exhibit superconducting behaviour, i.e. they have no electrical resistance at low temperatures. With all this research activity, the future looks promising for this remarkable molecule.