Molecular Möbius Strips and Trefoil Knots
With the current interest in so called "nanoscale" devices with
dimensions reaching down to molecular sizes, there exists a
fascinating boundary between the shapes that materials
(i.e. a large collection of molecules) and molecules
themselves can adopt. Understanding how molecular building
blocks can be used to construct nanoscale devices is the key to
understanding nanotechnology. A second boundary exists,
between idealised shapes, or topologies, defined by
mathematical equations, and the actual shapes adopted by the
collections of atoms (nuclei and electrons) we call molecules.
The Trefoil Knot and the Möbius Strip are
two such topologies which span both boundaries. Here we tell
the story from the molecular and electronic perspective. To
view the molecular models, you will have to install a
program capable of viewing molecule coordinate files.
The Boundary with Mathematics

 As a mathematical concept,
trefoil knots have many fascinating properties (see the Wolfram MathWorld
page from which the adjacent diagrams are taken). The one
we concentrate on here is that the trefoil knot also has the
property of being a Möbius strip. This
topology was first proposed by August Möbius in the 19th Century, and is
easily constructed by cutting a closed band into a single
strip, giving one of the two ends thus produced a half twist,
and then re-attaching the two ends. The mathematical properties of
Möbius strips are both fascinating and complex, but of
equal interest (to a chemist!) is whether a molecule or a
nanoscale material can be induced to adopt such a shape, and if
so, what its properties might be.
As a mathematical concept,
trefoil knots have many fascinating properties (see the Wolfram MathWorld
page from which the adjacent diagrams are taken). The one
we concentrate on here is that the trefoil knot also has the
property of being a Möbius strip. This
topology was first proposed by August Möbius in the 19th Century, and is
easily constructed by cutting a closed band into a single
strip, giving one of the two ends thus produced a half twist,
and then re-attaching the two ends. The mathematical properties of
Möbius strips are both fascinating and complex, but of
equal interest (to a chemist!) is whether a molecule or a
nanoscale material can be induced to adopt such a shape, and if
so, what its properties might be.
As it happens, two somewhat different kinds of molecular
Möbius strip can be envisaged; those where the nuclear
framework itself is twisted into a Möbius strip and those
where only the electronic component of the molecule, described
by a wavefunction, has this property.
Molecules with Nuclear Möbius strips
 The first step to
constructing a Möbius strip using molecules is to create
the molecular equivalent of a strip of paper. This is done by
starting with benzene, and extending it by
adding a series of benzo groups to turn it into napthalene,
anthracene and so on, forming what is called a poly-acene. The
two ends of this strip are then twisted and joined to form what
is called a Möbius cyclacene. Models for such systems
containing one, two or three twists can be
constructed.1 Although these molecules contain far
too much strain induced by the twisting to be likely to be
actually made, their properties can be modelled using quantum
mechanics. Unlike a true Möbius strip, where the "twist"
is evenly distributed along the entire length of the ring (see
the Mathworld models) these
molecular strips appear to "localise" the twist to a relatively
small region of the molecule. The implications of this
unexpected property are still being investigated.
The first step to
constructing a Möbius strip using molecules is to create
the molecular equivalent of a strip of paper. This is done by
starting with benzene, and extending it by
adding a series of benzo groups to turn it into napthalene,
anthracene and so on, forming what is called a poly-acene. The
two ends of this strip are then twisted and joined to form what
is called a Möbius cyclacene. Models for such systems
containing one, two or three twists can be
constructed.1 Although these molecules contain far
too much strain induced by the twisting to be likely to be
actually made, their properties can be modelled using quantum
mechanics. Unlike a true Möbius strip, where the "twist"
is evenly distributed along the entire length of the ring (see
the Mathworld models) these
molecular strips appear to "localise" the twist to a relatively
small region of the molecule. The implications of this
unexpected property are still being investigated.
Molecules with Electronic Möbius strips
To twist just the electrons in a molecule into a Möbius
strip requires some way of persuading them that it is in their
(energetic) interest to do so. The story of how this can be
done dates back to another August, this time August Kekulé. In
middle of the 19th century he suggested that the then recently discovered (and
unexpectedly stable) benzene molecule had a structure
consisting of a cyclic arrangement of the atoms. Following
suggestions by the discoverer of the electron J. J. Thompson (Phil.
Mag, 1921, 41, 510) and
E. C. Crocker (J. Am. Chem. Soc.,, 1922, 44,
1618) and --> Armit and Robinson (J. Chem. Soc., 1925,
1604) of the special significance of an "aromatic sextet", Erich Hückel
developed theories of molecular quantum mechanics which were
eventually used to show that such cyclic molecules, known as
aromatics, were particularly stable when 2,6,10,14,18 etc
(generally 4n+2, where n is zero or a positive integer)
electrons occupied a cyclic and parallel overlapping
arrangement of atomic p-orbitals (the "Hamburger" model. The
two buns can be thought of as representing the electrons in the
molecule and the meat patty the nuclei). The resulting
electronic stability imparts to such molecules a property known
as aromaticity, of which benzene remains the best known
example. In 1964 Edgar
Heilbronner2 worked out that cyclic molecules
with 4,8,16,20 etc (generally 4n) electrons could be equally
aromatic, but only if a twist was given to the otherwise
parallel overlap of the p-orbitals, a twist which corresponds
exactly to the Möbius twist! Amazingly, it has taken some
40 years for chemists to actually propose candidates for such
molecules. Some of the newly discovered (or invented)
Möbius electronic systems include: 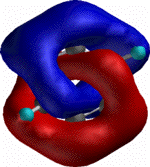 These molecules all exhibit (see right) a cyclic
Möbius-like twisting of the electrons (or more accurately,
of the electronic wavefunction).
These molecules all exhibit (see right) a cyclic
Möbius-like twisting of the electrons (or more accurately,
of the electronic wavefunction).
The last example above, in which three twisted rings were
joined at a single atom, brings us to the mathematical shape
known as the Trefoil Knot. By imagining a molecular chain of 36
carbon atoms, but this time not joining it at a central atom, a
molecular Trefoil knot can be constructed. Such an
isomer of carbon, were it possible to make, would join the list
of known carbon allotropes such as graphite, diamond and buckyballs.
A more realistic chemical model can be made by attaching benzo
groups to the outside to make a hydrocarbon (benzo groups
are derived from the molecule that started this story,
benzene). This "invented" molecular trefoil knot has both the
characteristics of a nuclear and an electronic
Möbius strip.
Nature is, unsurprisingly, ahead of us in invention.
Although they are only nuclear and not nuclear-electronic
Möbius strips, some molecules have indeed been persuaded
to wind themselves into a trefoil
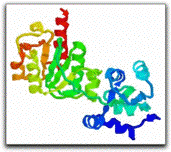
knot!6 Nature is also capable of knotting
proteins (large molecules or biopolymers, made of long chains
of connected amino acids). The active site
of one such protein (shown on the left) is constructed right on a trefoil
knot!7
The Boundary with Nanoscale materials
To complete the scaling up of this concept, some Japanese
workers have recently
reported8 that the inorganic conductor
NbSe3 can grow tiny crystals which take the form of
Möbius strips. The authors write at the end of their
article that "Our crystal forms offer a new route to exploring
topological effects in quantum mechanics as well as to the
construction of new devices", which could probably be said for
several of the other usual Möbius systems described here.
Thus an area of chemistry 180 years old, started with Faraday's discovery of benzene in
1825, continues into the 21st century!
Further Reading
- L. Turker, J. Mol. Struct (Theochem), 1998,
454, 83; S. Martin-Santamaria and H S. Rzepa, J.
Chem. Soc., Perkin Transactions 2, 2000, 2378-2381.
- E. Heilbronner, Tetrahedron Lett., 1964, 29,
1923.
- S. Martin-Santamaria, B. Lavan and H. S. Rzepa, J.
Chem. Soc., Perkin Trans 2, 2000, 1415; C. Castro, C. M.
Isborn, W. L. Karney, M. Mauksch, and P. von Rague Schleyer,
Organic Letters, 2002, 4, 3431-3434.
- D. Hall, N. Sanderson and H. S. Rzepa, Org. Biomol.
Chem., submitted for publication.
- D. Hall and H. S. Rzepa, Org. Biomol. Chem., 2003,
in press.
- A. M. Albrecht-Gary, C. O. Dietrich-Buchecker, J.
Guilhem, M. Meyer, C. Pascard and J. P. Sauvage,
Rec.Trav.Chim.Pays-Bas, 1993, 112, 427.
- O. Nureki, M. Shirouzu, K. Hashimoto, R. Ishitani, T.
Terada, T. Tamakoshi, T. Oshima, M. Chijimatsu, K. Takio, D.
G. Vassylyev, T. Shibata, K. Y. Inoue, S. Kuramitsu and S.
Yokoyama, Acta Cryst., Sect. D: Biological
Crystallography, 2002, D58, 1129-1137.
- S. Tanda, T. Tsuneta, Y. Okajima, K. Inagaki, K. Yamaya
and N. Hatakenaka, Nature, 2002, 417, 397 -
398.
(C) Henry S.
Rzepa, December, 2002 - May 2003.

 As a mathematical concept,
trefoil knots have many fascinating properties (see the Wolfram MathWorld
page from which the adjacent diagrams are taken). The one
we concentrate on here is that the trefoil knot also has the
property of being a Möbius strip. This
topology was first proposed by August Möbius in the 19th Century, and is
easily constructed by cutting a closed band into a single
strip, giving one of the two ends thus produced a half twist,
and then re-attaching the two ends. The mathematical properties of
Möbius strips are both fascinating and complex, but of
equal interest (to a chemist!) is whether a molecule or a
nanoscale material can be induced to adopt such a shape, and if
so, what its properties might be.
As a mathematical concept,
trefoil knots have many fascinating properties (see the Wolfram MathWorld
page from which the adjacent diagrams are taken). The one
we concentrate on here is that the trefoil knot also has the
property of being a Möbius strip. This
topology was first proposed by August Möbius in the 19th Century, and is
easily constructed by cutting a closed band into a single
strip, giving one of the two ends thus produced a half twist,
and then re-attaching the two ends. The mathematical properties of
Möbius strips are both fascinating and complex, but of
equal interest (to a chemist!) is whether a molecule or a
nanoscale material can be induced to adopt such a shape, and if
so, what its properties might be. The first step to
constructing a Möbius strip using molecules is to create
the molecular equivalent of a strip of paper. This is done by
starting with
The first step to
constructing a Möbius strip using molecules is to create
the molecular equivalent of a strip of paper. This is done by
starting with 
