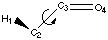 |
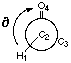 |
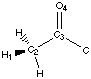
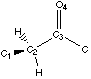
Figure 4.1 : Definition of the Dihedral Angle
A H-Bond exists between a functional group A-H and an atom or a group of atoms B in the same or a different molecule when (a)there is evidence of bond formation (association or chelation), (b) there is evidence that this new bond linking A-H and B specifically involves the hydrogen atom already bonded to A
We want to analyse hydrogen bonds with a proton on a carbon alpha to a carbonyl group or an other related group. The position of the proton is defined by a dihedral angle involving the carbonyl group (Figure 4.1). We want to compare calculations and experimental data. Calculations let us model the ease of creating hydrogen bonds as a property of the fragment (a partial charge on the proton). Experimental data shows the different dihedral angles for compounds in a database that contain such hydrogen bonds. We will first describe the method that can be used to measure the correlations, and then we will try to apply this method to the hydrogen bond we are are looking at.
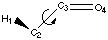 |
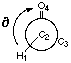 |
Figure 4.1 : Definition of the Dihedral Angle
| Number of compounds = f (parameter) | (1) |
| Property = g (parameter) | (2) |
| Number of compounds = h (property) | (3) |
| Number of compounds = h o g (parameter) | (4) |
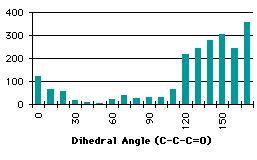
Several searches in a crystallographic database (the Cambridge Structural Database[27]) have been carried out. The requests specified that the distance between the hydrogen and the oxygen that form the hydrogen bond should be less than 2.3 Å (there is a Van de Waals contact when the distance is less than 2.6 Å, so we are sure that there will be a bond). Sheme 4.2 shows the results of this search.
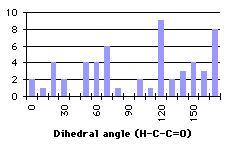 |
Graph 4.2 : Number of compounds with hydrogen bond
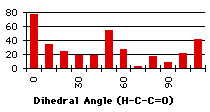
At a first glance, we cannot find any correlation between the search and the partial charges. We wonder if we have to take into account the energy as a function of the dihedral angle to refine this analysis. Another source of apparent disorder in the results of the searches appears with the distinction between the different types (primary, secondary or tertiary) of the carbon holding the proton involved in the hydrogen bond. Unfortunately the shape of the graphs describing the energies and the partial charge on the proton as a function of the dihedral angle depends on the types of the carbon alpha to the carbonyl. We will make a review of the different kinds of groups, trying to correlate the dihedral angle distribution found in the database with the energies and the partial charge on the hydrogen.
 |
 |
Primary |
 |
 |
Secondary |
 |
 |
tertiary |
Graph 4.3
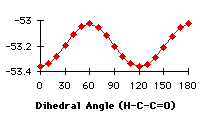
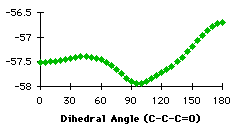
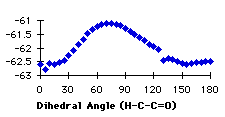
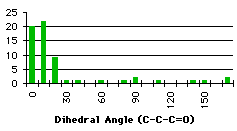
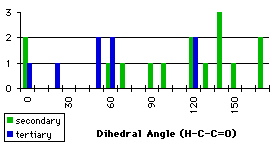
The first atom of the dihedral angle in the second structure is a carbon, because it is smpler to describe the variation in this way. The minimum at 100° corresponds to two different dihedral angles for the hydrogens (-140° and -20°). We have the confirmation that we have to handle the different groups separately. We have searched the database for compounds containing the groups described in graph 4.3. In each case we specified that the atoms in the fragment may be linked to each other only by bonds given explicitly. This command (no links) avoided strained cycles.
 |
Graph 4.4 : Number of compounds with secondary carbon
The results for the group with a secondary carbon are opposite to those we expected. Most of the compounds have a dihedral angle between 120° and 180°. Examining some of these compounds, we observed that they contain five or six membered rings that force the dihedral angle to be close to 120° or 180° (Figure 4.2)
 |
 |
| 6-members rings = ± 120° |
5-members rings = ± 180° |
Figure 4.2 : Effects of rings on the dihedral angle
We have to reject the compound with these motifs. Therefore we use another command (no cyclic route) to specify that the selected atoms in the fragment may not be connected by a cyclic bond.
 |
 |
Primary |
 |
 |
Secondary |
 |
 |
tertiary |
Graph 4.5
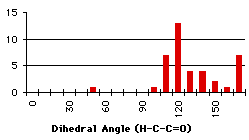
When the utmost has been done to eliminate external causes of specificity in the repartition of the compounds, only a few molecules exhibit all the conditions to be good candidates to a repartition due only to energetic factors. Unfortunately, the synthetic results of the search (the repartition of the dihedral angles) do not have the same shape as the calculation results. At first glance, the correlation seems to be better with the primary carbon alpha to the carbonyl and we want to measure it. We will model a linear dependance of the number of compounds with the heats of formation (Graph 4.3). We will use eleven linear functions, with a simple principle of construction : the relative weights of the minimum and maximum heats of formation go from (1,0) to (1,1)*. This describes all the expected range of linear behaviours. Hereafter, the linear functions are multiplied by a coefficient that will give the same total number of compounds as we get from the search. The correlations found with the ![]() 2 are very small (<<10-10). To simulate exponential functions we made the same calculation with linear functions with respective weights for minimum and maximum going from (1,0) to (1,-1) and when the value of the function was negative, it was set to zero. Even in this case the correlations were very bad. The main problem is that the 'noise' is too important (the peak at 80° for instance) and there are probably some external factors that we have not taken into account (the large peak at 60°).
2 are very small (<<10-10). To simulate exponential functions we made the same calculation with linear functions with respective weights for minimum and maximum going from (1,0) to (1,-1) and when the value of the function was negative, it was set to zero. Even in this case the correlations were very bad. The main problem is that the 'noise' is too important (the peak at 80° for instance) and there are probably some external factors that we have not taken into account (the large peak at 60°).
 |
Graph 4.6 : Number of compounds with hydrogen bond (secondary and tertiary carbons)
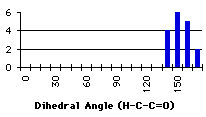
With a methyl group (primary carbon alpha to the carbonyl), the search was extended to a maximum distance of 2.5Å. It is obvious that there are two main peak (graph 4.7). A detailed review of the compounds corresponding to these two peaks reveals that they belong to the same classes (Figure 4.3). The 120° peak correspond to a conjugated diketone where two hydrogens are forming a weak hydrogen bond: the oxygen-proton distance in the search result was between 2.3Å and 2.5Å. This other class of compound corresponds to a methyl ketone in a position ortho to an oxygen. The distance here is between1.8Å and 2.3Å.
 |
Graph 4.7 : Number of compounds with hydrogen bond (primary carbons)
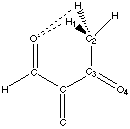 | 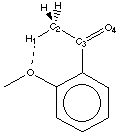 |
Figure 4.3
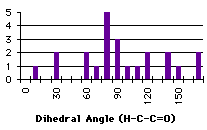 |
Graph 4.8 : Number of compounds with hydrogen bond (Ester)
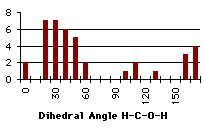 |
Graph 4.9 : Number of compounds with hydrogen bond (alcohol)
We could have searched the database to find compounds containing our fragment and an oxygen atom (or any other electronegative atom) without looking any geometrical property. Then we should have retrieved the shape of the crystal cell and carefully examined it to see if there is a hydrogen bound. This process implies that we have to write some programs to handle intermolecular hydrogen bonds.
We are now aware of the different external factors that could occur in a statistical analysis on a chemical database. There are a lot of compounds but there are not so many different motifs. If our sample do not contain enough molecules, we may often encounter the same motifs and our analysis become meaningless.
| a(r).H°min + b(r) = 1 | (5) | |
| a(r).H°max + b(r) = r | (6) | |
| r = 0, 0.1, 02, ... , 0.9, 1 | (7) |
| Previous | Table of Contents | Next |