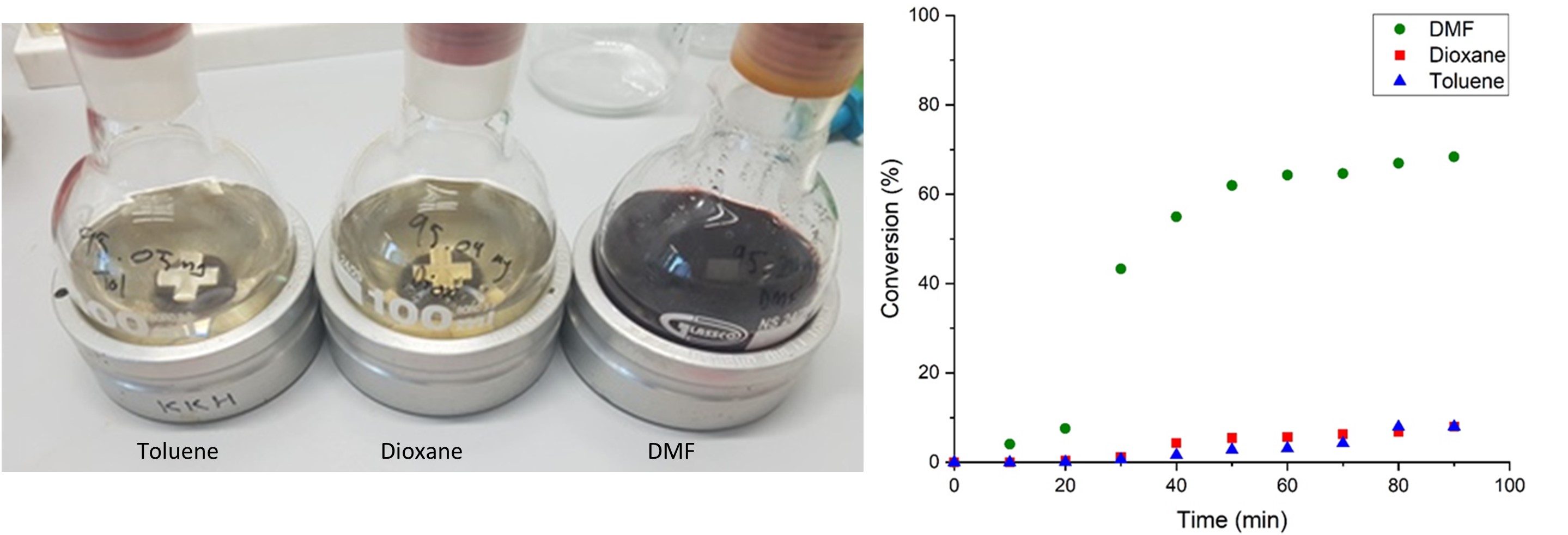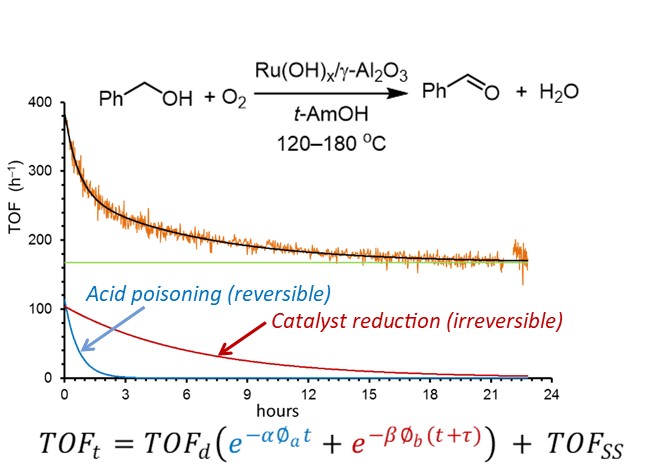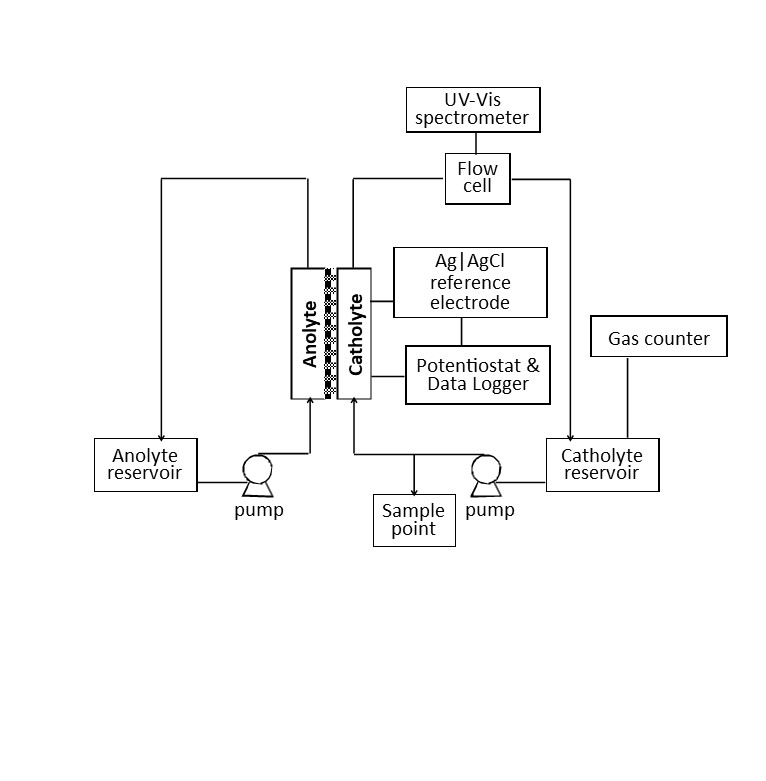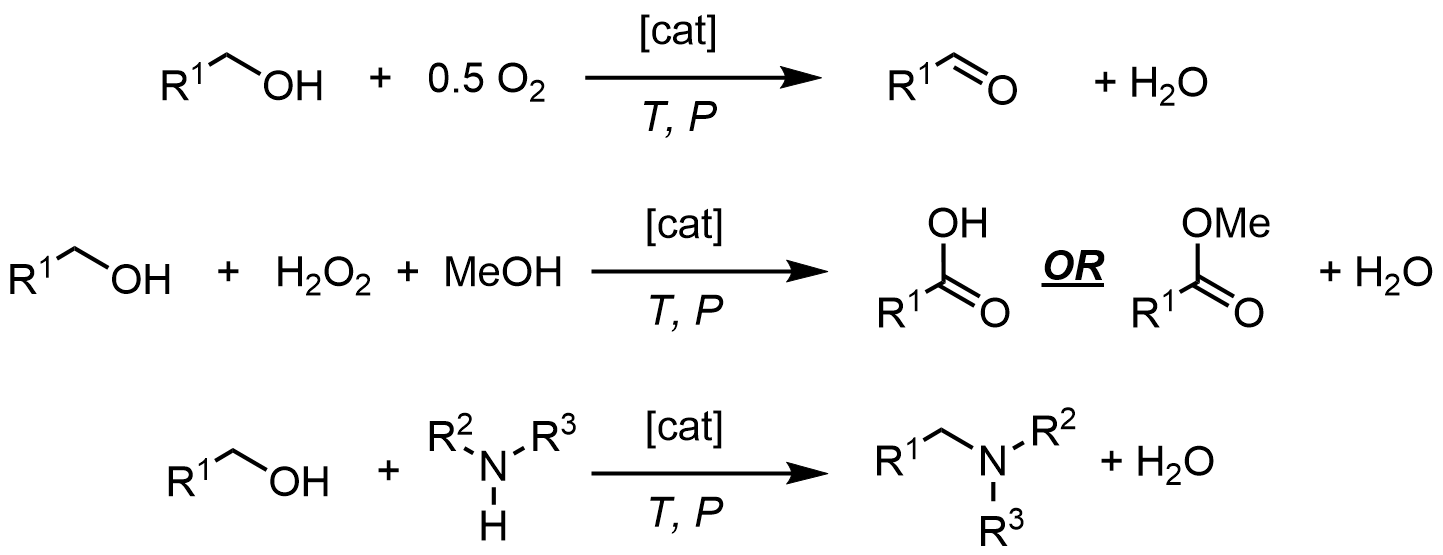
Utilizing alcohol feedstocks in continuous flow
Alcohols are readily available with great structural diversity from renewal sources, making them attractive feedstocks for the production of high-value chemicals, such as selective oxidation to aldehydes/ketones, acids/esters. Alcohols can also be used to selectively alkylate amines (including ammonia), without extraneous reagents, generating only water as a benign byproduct. Commercially-available Ru, Au and Ni catalysts were utilised in these reactions and we demonstrated that better selectivity can be achieved in continuous flow.