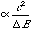
Theoretical calculations were carried out in the gas phase at the restricted Hartree-Fock level (RHF) level using the PM3 and AM1 semi-empirical SCF-MO methods, as implemented by the CacheMOPAC v.94.1 program[35] All structures were optimised using the eigenvector following algorithm, followed by a vibrational analysis to characterise the transition state.
A search for organics only with a non-bonded contact between a hydrogen alpha- to a carbonyl and an ether, hydroxyl or carbonyl oxygen between 1.5 and 2.0 A. in the April 1995 release of the Cambridge Crystallographic Database[15] was carried out using version 5.09 of the Quest software. Fourteen entries fulfilled the close contact criteria
Theoretical calculations on the transition states docked into the beta-lactamase were attempted using QUANTA 4.0[32] as a CHARMm[33] interface. Calculations were attempted in the RTF mode; the forcefield parameters were customised to account for the novel atom types and O-C bond of the transition state. The file defining atom types, MASSES.RTF, the CHARMm parameter file PARM.PRM and the QUANTA display parameter file param.par were all customised to define two new atom types, OTS and CTS. These corresponded to the Ser70 oxygen and the carbonyl carbon of the transition state respectively, as these are both imbetween sp2 and sp3 hybridisation and not satisfactorily described by any of the standard QUANTA[32] atom types. The force constant for the transition state bond was also defined as being extremely high to prevent further minimisation of the transition state bond length as calculated by MOPAC[35].
Theoretical calculations on the transition states docked into the -lactamase were also attempted using MACROMODEL 4.0[34].
1. K. Iseki, T. Nagai and Y. Kobayashi, Tetrahedron Lett., 1993, 34, 2169; R. W. Lang, Drug
News and Perspectives, 1991, 4, 13; G. L. Hann and P. Sampson, J. Chem. Soc. Chem.
Commun., 1989. 1650; E. Differding and R. W. Lang, Tetrahedron Lett.., 1988, 29, 6087.
2. D. O'Hagan, N. A. Zaidi and R. B. Lamont, Tetrahedron Asymmetry, 1993, 4, 1703; D.
O'Hagan and N. A. Zaidi, J. Chem. Soc. Perkin Trans I, 1992, 947; T. Yamazaki, N. Okamura
and T. Kitazume, Tetrahedron Asymmetry, 1990, 1, 521; T. Yamazaki, T. Ohnogi and T.
Kitazume, Tetrahedron Asymmetry, 1990, 1, 215; J. T. Lin, T. Yamazaki and T. Kitazume, J.
Org. Chem., 1987, 52, 3211; T. Kitazume and J. T. Lin, J. Fluorine Chem.., 1987, 34, 461.
3. T. Fujisawa, I. Ichikawa and M. Shimizu, Tetrahedron Asymmetry, 1993, 4, 1237.
4. P. Kalaritis, R. W. Regenyne, J.J. Partidge and D. L. Coffen, J. Org. Chem., 1990, 55, 812; T. Kitazume, K. Murata and T. Ikeya, J. Fluorine Chem., 1986, 31, 143.
5. David O'Hagan and Henry S Rzepa,J . Chem. Soc., Perkin Trans 2, 1994, 3
6. . L. Dasaradhi and D. O'Hagan, Bio. Med. Chem. Letts., 1993, 3, 1655.
7. S.S. Wong and M. N. Paddon-Row, J. Chem. Soc. Chem. Commun., 1990, 456; N. T. Anh
and O. Eisenstein, Nouv. J. Chem, 1977, 1, 61.
8. O. Casher, D. O'Hagan, C. A. Rosenkranz, H. S. Rzepa and N. A. Zaidi, J. Chem. Soc.,
Chem. Commun, 1993, 1337; M. S. Baird, J. R. Al Dulayymi, H. S. Rzepa and V. Thoss, J.
Chem. Soc., Chem. Commun, 1992, 1323; B. Halton, R. Boese and H. S. Rzepa, J. Chem. Soc.,
Perkin Transactions 2, 1992, 447
9. M. J. S. Dewar, Enzyme, 1986, 36, 8; W. N. Lipscomb, Acc. Chem. Res., 1982, 15, 232
10. Frontier Orbitals and Organic Chemical Reactions, Ian Fleming, Wiley, 1976
11. The energy gained and lost when orbitals of one reactant overlap with
another
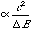
The Klopman Salem equation[10] states that:
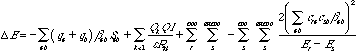
where qa and qb are the electron populations in atomic orbitals a and b
and S are the resonance and overlap integrals respectively
Qk and Ql are the total charges on atoms k and l
is the local dielectic constant
Rkl is the distance between k and l
cra is the coefficient of atomic orbital a in molecular orbital r; r refers to the MO's on one molecule, s refers to those on another.
Er is the energy of molecular orbitals
12. Calculated difference in activation energy lipase: 2.4 kcal mol-1; calculated activation energy difference for beta-lactamase: 4.46 kcal mol-1 calculated using the PM3 hamiltonian
13. Calculated free energy difference lipase: 2.5 kcal mol-1, calculated free energy difference beta-lactamase: 2.43 kcal mol-1, both calculated using the PM3 hamiltonian.
14. Calculated entropy difference lipase: 0.4 calK-1mol-1; calculated entropy difference beta-lactamase: -6.78 cal K-1 mol-1, both calculated using the PM3 hamiltonian
15. F. H. Allen, J. E. Davies, J. J. Galloy, O. Johnson, O. Kennard, C. F. Macrae, E. M. Mitchell, G. F. Mitchell, J. M. Smith and D. G. Watson, J. Chem. Inf. Comput. Sci., 1991, 31, 187.
16. J.Bode,H.Schenk, Cryst.Struct.Commun., 645, 6,1977
17. G.Reck,A.Barth, Cryst.Struct.Commun., 1001, 10, 1981
18. W.F.Berkowitz, S.C.Choudhry, J.A.Hrabie, J.Org.Chem., 824, 47,1982
19.I.B.Levshin, M.M.Kaganskii, K.A.V'yunov, A.A.Tsurkan, A.I.Ginak, A.A.Espenbetov, A.I.Yanovskii, Yu.T.Struchkov, Zh.Prikl.Spektrosk, 103, 37,1982
20. A.M.Stern, B.M.Foxman, A.H.Tashjian Junior, R.H.Abeles, J.Med.Chem., 544, 25, 1982
21. C.Pascard-Billy, Bull.Soc.Chim.Fr., 2293, 1962
22. R.Popovitz-Biro, C.P.Tang, H.C.Chang, M.Lahav, L.Leiserowitz, J.Am.Chem.Soc., 4043, 107,1985
23. P.D.Leeson, J.C.Emmett, V.P.Shah, G.A.Showell, R.Novelli, H.D.Prain, M.G.Benson, D.Ellis, N.J.Pearce, A.H.Underwood, J.Med.Chem, 320, 32, 1989
24. Y.Nawata, Y.Ishitani, I.Matsuura, H.Oishi, K.Ando, Acta Cryst.,C (Cr.Str.Comm.), 1112, 45, 1989
25. R.E.Ireland, W.J.Thompson, N.S.Mandel, G.S.Mandel, J.Org.Chem., 3583, 44, 1979
26. T.Uechi,Y.Fuchita, Bull.Chem.Soc.Jpn., 2831, 65, 1992
27. M.Massias, S.Rebuffat, L.Molho, A.Chiaroni, C.Riche, B.Bodo, J.Am.Chem.Soc., 8112, 112, 1990
28. D.H.G.Crout, C.R.McIntyre, N.W.Alcock, J.Chem.Soc.,Perkin Trans.2, 53,1991
29. J.Seetharaman, S.S.Rajan, Acta Cryst.,C (Cr.Str.Comm.), 1714, 48, 1992
30. Calculated entropy difference between stereoisomers is -6.78 or 0.53 cal mol-1
31. C.C.H.Chen,J.Rahil,,R.F.Pratt,O.Herzberg, J.Mol.Biol, 234, 165, 1993
32. QUANTA 4.0, Molecular Simulations Inc.
33. B. R. Brooks, R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, M. Karplus,.J. Comp. Chem., 1983,4, 187
34. MACROMODEL 4.0, Columbia University, 1993
35. J. J. P. Stewart, Fujitsu Ltd., Tokyo, Japan, 1993.
Available from Quantum Chemistry Program Exchange, University of Indiana, Bloomington, IN.
Dr. H. S. Rzepa*, research supervisor, for all his help.
O. Casher*, C. Leach*, C. Page* for help with Macintosh and UNIX operating systems
J. W. Davids*; R.Scott, MSI Support, Cambridge; MSI Support, USA: Dr. M. Saqi, GlaxoWellcome for help with QUANTA 4.032
Richard Kinder* for help in conversion to HTML
*Imperial College of Science, Technology and Medicine