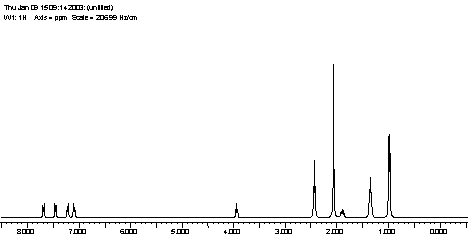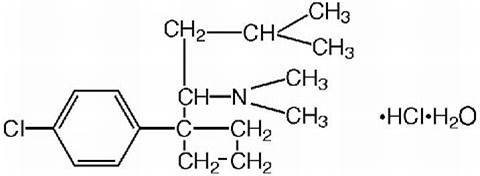
| Property | Value | Comments |
| Chemical name | (+/-)-Sibutramine | 16Reference: Beilstein online |
| Autoname | {1-[1-(4-chloro-phenyl)-cyclobutyl]-3-methyl-butyl}-dimethyl-amine | 16Reference: Beilstein online |
| Molecular Formula | C17H26Cl N | 16Reference: Beilstein online |
| Molecular Weight | 279.85 | 16Reference: Beilstein online |
| Optical Rotatory Power of the (+) enantiomer | 3.3o | Type - [alpha] Concentration - 1.5g/100ml Solvent - H2O Wavelength - 589nm 17Reference: Fang et al. |
| Melting Point | 191-192oC | Crystallised from H2O 18Reference: Jeffery et al. |