History of antifungal agents
The development of antifungal drugs was extremely slow despite the knowledge of their existence well before that of bacteria for instance, the first antibacterial agent, penicillin came into use in 1941 whereas the first antifungal agent, Nystatin was not discovered until 1949. This is very surprising since even in 1665, thrush caused by Candida albicans was classed as a fatal disease and it was not until 1835 that the first microorganism, Beauvaria bassiana was discovered to cause disease. The main reasons believed for the slow development of broad-spectrum agents is due to the nature of fungal diseases: they are eukaryotic species which are biochemically similar to human hosts as opposed to prokaryotic like bacteria. This means that developing a drug to combat fungal infections leaving the human host unaffected is very difficult. However, there has been an increase in the number of immunosuppressed cancer, HIV and organ transplant patients consequently increasing the number of opportunistic infections by fungi. Just after the Second World War the treatments available for fungal infections were weak acids and phenolic dyes. In the early 1960ís Griseofulvin, the first orally effective antibiotic was used for dermatophytosis management and later broad-spectrum agents arrived, with the first being iodinated trichlorophenol haloprogin, which functioned by disrupting the fungal cell membrane. 1969 heralded the discovery of azole antifungal agents. Imidazole agents such as Clotrimazole, miconazole, sulconazole and bifonazole acted by binding to cytochrome p-450, thus blocking ergosterol synthesis in the fungal cell membrane. Then came the devolpment of triazole antifungal agents that were less likely to cause heptotoxicity possibly due to their diminutive effects on cytochrome p-450-dependent enzymes. Terconazole was the first triazole developed, followed by itraconazole and finally fluconazole.
Development of fluconazole
Before the development of fluconazole the two major antifungal agents
available were amphotericin B and flucytosine. The problems with these
agents was that amphotericin B has to be given intravenously and had severe
adverse effects, whereas flucytosine was orally active but had a limited
range of action and resistance was easily developed.
In 1978 Pfizer based in Sandwich, Kent started a programme to find
an agent to improve on the available medicines culminating in the creation
of fluconazole, 2-(2,4-difluorophenyl)-1,3-bis (1H-1,2,4-triazol-1-yl)-2-propanol.
The characteristic list required that the agent had to be
Known azole antifungals main disadvantage was that they were metabolised
and highly lipophilic which meant that they were not effective orally.
Janssen Pharmaceutica announced in mid-1978 that they had prepared ketoconazole,
an imidazole derivative, which was proven to be orally effective but was
still degraded metabolically
The avenue Pfizer pursued therefore was a variety of compounds similar
in structure to ketoconazole. They eventually replaced the imidazole
unit with 1,2,4-triazole derivative as they still hadnít overcome the susceptibility
to metabolism - it was more active in vivo but four times less potent in
vitro.
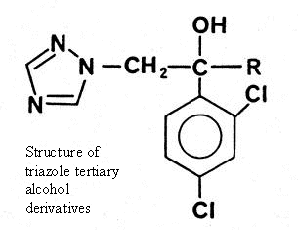
Consequently, attention was turned to the triazole tertiary alcohol
derivatives in which the R substituent was varied, in order to obtain
compounds resistant to metabolism and of low lipophilicity.
