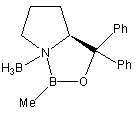
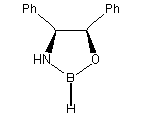
Catalysts:
 |
 |
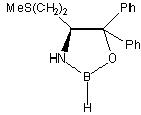 |
| Corey & Merck group catalysts |
Pfizer catalyst | Martens catalyst |
Steriospecific Synthesis
The previously described synthesis routes all lead to racemic mixtures of products. However, recent demand has increased for the (R)-isomer of salbutamol, as it is known that the (S)-isomer has no beneficent effect, and its presence may even decrease the effectiveness of racemic salbutamol. Whilst it is possible to obtain the (R)-isomer through resolution of a racemic mixture, this complicates the industrial manufacturing process. Therefore, a number of synthetic routes have been researched for synthesising (R)-salbutamol. Of these, one of the most promising, due to its high yields and small number of steps is outlined below. A number of chiral catalysts can be used in this reaction.
| Chiral Catalysts: |
|
Step 1:
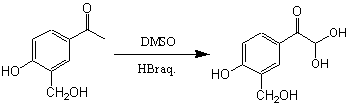 |
Yield : ~80% |
Step 2:
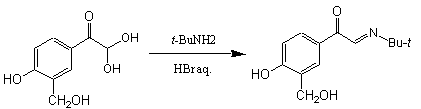 |
Yield : 75-95% |
Step 3:
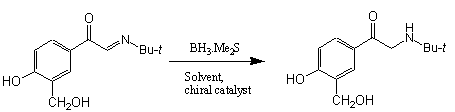 |
Yield : >89%% |
Varying the temperature at which the reaction is carried out and the catalyst concentration affects which isomer is formed, although this also affects the yield of each isomer. At higher temperature and higher catalyst concentration, the (S)-isomer is preferred, the product being nearly 100% enantiomerically pure. At lower temperature and lower catalyst concentration, the (R)-isomer is favoured.