Synthetic Route 2: A Stereospecific Approach.
Stereospecific Synthesis of (Z) - Tamoxifen via carbometalation of Alkynylsilanes.
Studied for historical reasons rather than synthetic brilliance. This synthesis was the first stereo specific synthesis of (Z) Trans Tamoxifen. Comparison between this synthesis and the previous route I believe can illustrate the development of synthetic approaches to large molecules. In particular the quest for stereo specific reactions. So starting from an alkynylsilane (A) and through a series of reactions we can generate only the (Z) - Trans isomer of Tamoxifen.
Again for ease of understanding the complete synthesis has been broken down into a number of steps.
Step1.
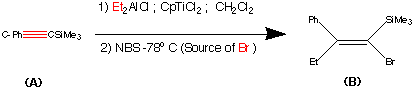
Step1.
This step contains the vital stereo specific step. Namely the carbometalation of the alkynylsilane.It is this step which establishes the stereochemistry about the double bond. The phenyl (trimethyl silyl) - acetylene was carbometalated with diethylaluminium chloride - titanocene dichloride reactant to produce an organometallic intermediate. This organometallic intermediate was then cleaved with N bromosucciniamide to produce the alkene (B) in 85% yield.
The stereochemistry was assigned as E (Cis) mechanistic evidence suggests that this is linked to some steric reasons.
(Earlier work dedicated to this reaction see : Miller, R.B. Al-Hassan.M.I J.Org.Chem. 1984, 49, 725)
Step2.
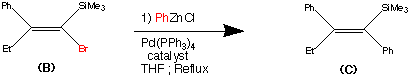
Step 2.
The second step shows the stereo specific replacement of the Br group by a phenyl group. This was achieved by use of Palladium - catalysed coupling of compound (B) with phenyl zinc chloride to form (C) the vinylsilane in a 95% yield.
Step3.
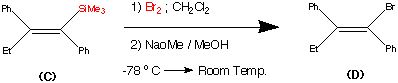
Step3.
This step during the synthesis was reported to be tricky and several approaches were attempted before a successful technique was discovered.
The objective of this step was to replace the trimethyl Silyl group by a suitable halogen atom (e.g. Bromine or Iodine)
However a facile reaction was reported when (C) was treated with bromine - sodium methoxide at -78°C to produce the vinyl bromide (D) in a yield of 85%
Step 4.
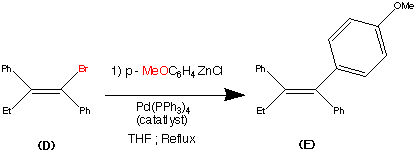
Step 4.
The vinyl bromide (D) coupled well with a Zinc organometallic species to produce (E) the ethyl triaryl olefin in a yield of 84%.
Step 5.
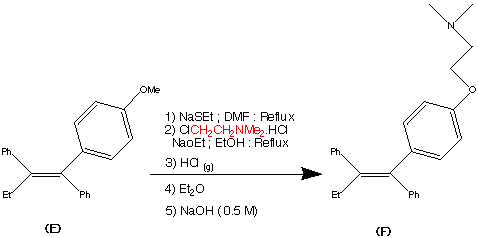
Step 5.
The formation of (F) Tamoxifen was achieved by demethylation with sodium ethylthoilate in refluxing dimethyl formamide. then reaction of the phenoxide ion with 2-( dimethylamino)ethyl chloride via a SN2 substitution.
Purification of the crude product was achieved via it's hydrochloride salt ( via a reaction with HCl (g)) then F was regenerated by treatment with dilute base this produced the stereospecific (Z)- Trans isomer in an overall yield of 60%.
Comments.
This synthesis was a major advancement in the formation of the trans isomer. The key step which developed the stereospecific nature of the final product was included early in the synthesis. This stereochemistry was preserved throughout the later steps of the preparation.
|
Huw Tanner.Undergraduate Year 2 .Imperial College of Science,Technology and Medicine. CIT Project Study of Tamoxifen. |
|
