Biological activity of Tamoxifen.
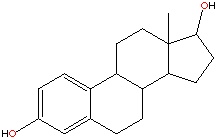
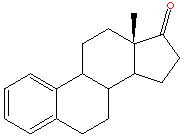
Oesterogen is a steroid and there has been lots of success in the field of cancer research with the development of drugs built around the steroid nucleus.(Some examples are shown below.) These have reduced the oestrogen activity by again binding to the oestrogen receptor sites.However in this respect tamoxifen is a lot different from the other drugs used for this purpose. As such from a chemical description of tamoxifen it is most definitely not a steroid type drug.
Tamoxifen belongs to a family of compounds known as SERM's!
What is a SERM? I here you ask !
SERM stands for selective oestrogen receptor modulator .A SERM is a molecule that binds with high affinity to the estrogen receptor (ER) but has tissue-specific effects distinct from estradiol and estrogen itself.There are a large number of compounds that display mixed agonist / antagonist effects on the Oestrogen Receptor, including:
In fact tamoxifen is a non-steroidal triphenylethylene,this family of compounds have shown steroidal mimicking characteristics. Tamoxifen can adopt a structural conformation which resembles the steroid nucleus.Some steriod compounds related to treatment of breast cancer are shown below.
Hence in this conformation Tamoxifen can sit in a steroidal membrane and effectively block off the oestrogen receptor sites. Tamoxifen competitively inhibits the binding of oestrogen to the oestrogen receptor sites. As well as Tamoxifen directly blocking the oestrogen receptor sites there is also another important mechanism by which Tamoxifen can inhibit growth of cancerous cells. In the body Tamoxifen is metabolised , this is thought to be due to a reaction of Tamoxifen with ovarian carcinovia cells (BG-1). These cells possess the ability to hydroxylate the geminal phenyl ring and produce a Tamoxifen derivative 4-Hydroxytamoxifen. This is an example of a internal bodily reaction of which there are thousands.Most of which are going on as I am typing this! This derivative of Tamoxifen is believed to be a stronger oestrogen inhibitor by a factor of almost 500! this can be rationalised to the structure of 4-Hydroxytamoxifen. The 4-Hydroxytamoxifen possess an - OH group , the oxygen of which has two lone pairs of electrons. It is though that these lone pairs can effectively improve the binding of the derivative to the oestrogen receptor. This as in the case of Tamoxifen has the effect of blocking the site and vastly reducing oestrogen receipting properties.
Further research also indicates that Tamoxifen has another mechanism of preventing reproduction of malignant cells. As Tamoxifen can intimitate a steroid nucleus it is also possible to initiated binding to other biological enzymes. In some cases Tamoxifen has been reported to bind to the enzymes which facilitate oestrogen hormone production. This effectively limits the oestrogen present in the system. This makes the oestrogen receptor sites redundant ( even if Tamoxifen fails to bind to them directly) and again has the desired effect of reducing the oestrogen activity in the body.The evidence of such a mechanism is however not well documented. Research into this aspect of tamoxifen mechanistic is still in it's early stages.
As well as this derivitive which is formed by interal bioloagiocal reactions lots of research has been aimed at producing synthetic derivtives of Tamoxifen.It is hoped that these derivitves will show binding affinities greater than that of Tamoxifen.

|
Huw Tanner.Undergraduate Year 2 .Imperial College of Science,Technology and Medicine. CIT Project Study of Tamoxifen. |
|