2003: Organic Problems Set 2
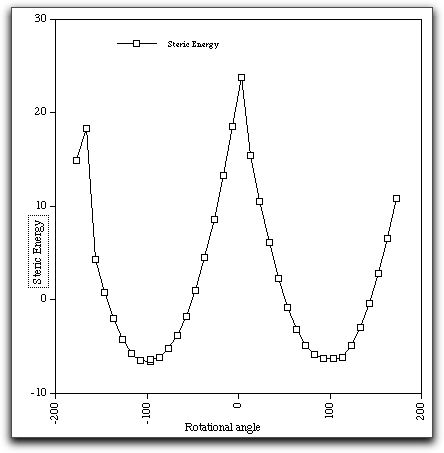
The modelling was done with the Chem3D program, using the MM
energy function and the "dihedral driver" option. The MM2 mechanics field
models the steric interactions and a simple Huckel calculation
models the resonance effects. Note the "cusps" at around 0 and
180 degrees. Probably what needs to happen is the N-C=O amide
bond must also rotate to some extent to avoid the worst of the
steric repulsions. The "dihedral driver" geometry definition
used to create this energy profile does not model this other
bond rotation very well, and hence the occurance of "cusps".
Nevertheless, it does appear from this profile that the steric effects
quite overwhelm the pi resonance effects in this particular example. This
balancing of two opposing effects is typical of many issues of selectivity
in organic chemistry, and only quantitative models can predict the eventual
outcome.
Question 2
This is an application of the "rabbit ear" way of looking at
lone pairs on oxygen atoms. Where the possibility for adjacent
stereoelectronic interaction is with a C-O sigma-* orbital, the most
appropriate hybridisation of the two lone pairs is the "tetrahedral"
or "rabbit ear" mode. This hybridisation ensures optimum overlap with the
C-O sigma-* system. This contrasts with the previous example, where the oxygen
was interacting with the more complex C=O system, which contains both pi and sigma
components. Under these circumstances, the two oxygen lone pairs are best hydridised
into a pure p lone pair (suitable for overlap with the C=O pi system)
and into one sp2 lone pair (suitable for overlap with the C=O sigma-*
system). The tricky decision of course is knowing when to use "rabbit ear"
hybridisation and when to use the sigma/pi hybridisation. Ultimately, only
the "variation principle" in quantum mechanics will allow a decision as to which
leads to the lowest energy.
Tutorial Questions and Models
A set of tutorial questions and answers is available.
Qu 1
Qu 2
Qu 3
