S vs O as a Neighbouring Group
In a first order analysis, a nucleophilic/electrohilic
interaction can be approximated by the interaction of two key
molecular orbitals; the HOMO (the nucleophile) donating a a
pair of electrons to the empty LUMO (the electrophile). This
interaction depends on:
- The energy gap between these two orbitals. The smaller
this gap, the better the interaction. Specifically, the
higher the energy of the HOMO (i.e the less negative) the better a nucleophile it
represents, whilst the lower the energy of the LUMO (i.e. less positive or more
negative) the
better the electrophile, and the combination of these two is
the HOMO-LUMO gap.
- The spatial overlap of the HOMO with the LUMO.
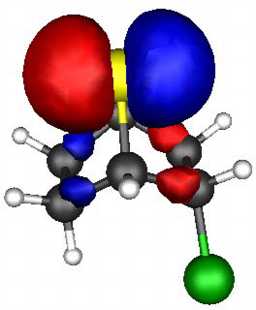
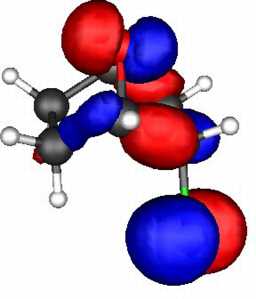
These two effects can be probed by a molecular orbital
calculation on the two reactants, for which acetolysis for the S system shown below
is observed to be 109 faster than for the O analogue.
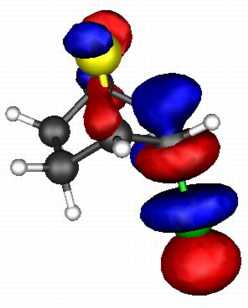
The results (ab initio 3-21G* level) are as follows.
- S bicyclic: HOMO-LUMO gap = 0.546 Hartree.
- O bicyclic: HOMO-LUMO gap = 0.606 Hartree.
The difference amounts to 157 kJ (1 Hartree = 2624 kJ).
The spatial overlap can be seen below. On both counts, S wins over O.
|
|
S bicyclic |
O bicyclic |
| LUMO |
 |
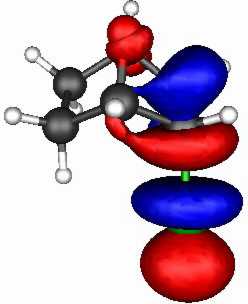 |
| HOMO |
 |
 |
Product Stability
One can test the above (reactant based) conclusions by calculating
the energy of the carbocation resulting from elimination of Cl-.
Thus the energy to create a cation from the O system is +980 kJ
whereas that for the S system is +898 kJ (AM1 calculations). Solvation and other
factors not withstanding, this difference of 82 kJ abundantly explains
the difference in solvation rates. Notice in particular that ring strain prevents
the O system from closing to form an oxonium ion, whereas the larger more flexible
S system does indeed so close.