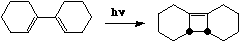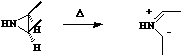5. Examples Of Electrocyclic Reactions.
Opening of the cyclopropyl cation involves only one electron arrow, and hence the number of
cyclically conjugated electrons is 2 (4n+2, n=0). Under thermal conditions, this will go via a Huckel
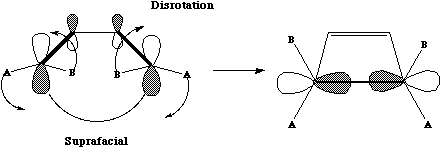
topology involving suprafacial components., which has the effect of rotating both methyl groups
outwards in opposite directions (disrotation).

Both the reactions below involve two electron arrows and hence are 4n systems (n=1).
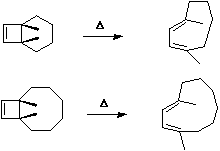
Thermally,
they will proceed via a Mobius topology involving one antarafacial component. Both methyl groups
will rotate in the same direction (conrotation) leaving one endocyclic and one exocyclic. This can
also be (optionally) illustrated via the Frontier orbital approach.
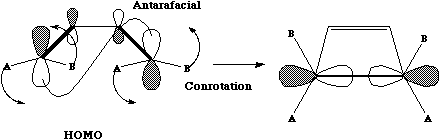
In
order to form a new sigma bond, the
HOMO of the butadiene fragment must rotate in the sense shown, one node coming from
above the plane of the molecule, the other from below (ie antarafacial);
Under photochemical conditions, a 4n reaction is predicted to proceed via a Huckel topology with
suprafacial bond formation, as for example;
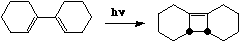
The sense of this can again be seen from the frontier orbital (normally the LUMO but in the excited
state occupied by a single electron, and hence effectively the HOMO).
Where heteroatoms are involved, the lone pair counts as two electrons. Thus the ring opening of
aziridine is a 4 electron process, hence 4n proceeding via Mobius topology with both methyl groups
rotating in the same direction (conrotation).
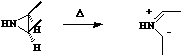
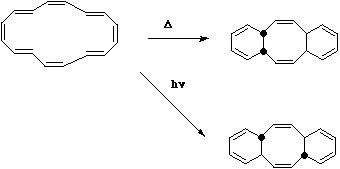
One important aspect when counting electrons is to include only the cyclically conjugated system. In
the example below, this consists of two independent six electron (4n+2) reactions, and the electrons
in the central double bonds are NOT counted
Similarly, carbanions contribute 2 electrons to the total count;
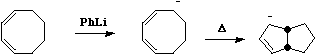
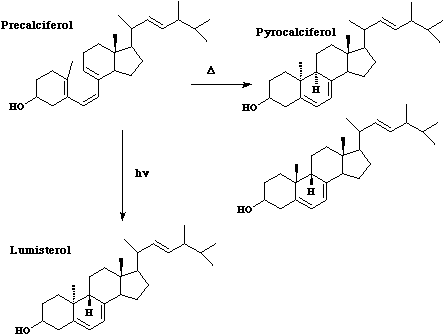
Also in this category are some examples of steroidal reactions which could only be rationalised on
the basis of the selection rules;
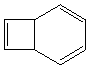
Finally, as a conundrum, can you decide if the following ring opening proceeds via a Huckel or a
Mobius transition state? (For disucussion in tutorial groups! Not examinable!).
Return to index