Principles
of NMR.
In
quantum mechanical terms, the nuclear magnetic moment of a nucleus can
align with an externally applied magnetic field of strength Bo in
only 2I+1 ways, either re-inforcing or opposing Bo. The energetically
preferred orientation has the magnetic moment aligned parallel with the
applied field (spin +1/2) and is often given the notation a,
whereas the higher energy anti-parallel orientation (spin -1/2) is referred
to as b.
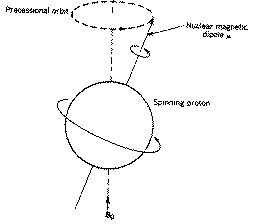
The rotational axis of the spinning nucleus cannot be orientated exactly
parallel (or anti-parallel) with the direction of the applied field Bo
(defined in our coordinate system as about the z axis) but must
precess about this field at an angle (for protons about 54š) with an angular
velocity given by the expression;
wo
= gBo
...(1) (the Larmor frequency, in Hz)
The
constant g
is called the magnetogyric ratio and relates the magnetic moment m
and the spin number I for any specific nucleus;
g
= 2pm/hI
...(2) (h is Planck's constant)
For a single nucleus with I=1/2 and positive g,
only one transition is possible (D
I=1, a single quantum transition) between the two energy levels;
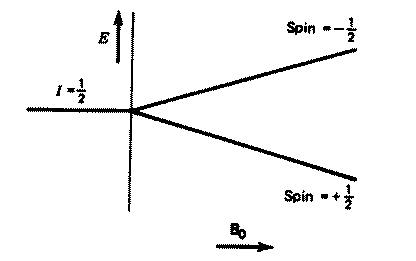
NMR is all about how to interpret such transitions in terms of chemical
structure. We will first consider the energy of a typical NMR transition.
If angular velocity is related to frequency by wo
= 2¼n,
then
n
= gBo/2¼
...(3)
It
follows that proton NMR transitions (DI=1)
have the following energy;
hn
= DE
= hgBo./2p
...(4)
For
a proton g
= 26.75 x 107 rad T-1 s-1 and Bo
~ 2T, DE
= 6 x 10-26 J. The relative populations of the
higher (n2) and lower (n1) energy levels at room
temperature are given by the Boltzmann law;
n2/n1
= e-DE/kT
~ 0.99999. ...(5)
For NMR, this means that the probability of observing a transition from n1 to n2 is only slightly greater than that for a downward transition, ie the overall probability of observing absorption of energy is quite small. This relationship also explains why a larger Bo favours sensitivity in NMR measurements, increasing as it does the difference between the two Boltzmann levels, and why NMR becomes more sensitive at lower temperatures.