Applications of 13C NMR.
To illustrate some of the
concepts developed above, we will first concentrate on Carbon. The most
abundant isotope 12C has no overall nuclear spin, having
an equal number of protons and neutrons. The 13C isotope
however does has spin 1/2, but is only 1% abundant. Carbon NMR spectra are
characterised by the following; * A chemical shift range of about 220 ppm,
normally expressed relative to the 13C resonance of
TMS.
* A natural linewidth of ca 1Hz, related to the values of the
relaxation times T1 and T2.
* A Larmor frequency in the range of 20-100
MHz, for typical spectrometers.
* Typically about 5-20 mg of sample
dissolved in 0.4 - 2 ml of solvent (normally CDCl3) are required, and a good
spectrum would be obtained in 64 - 6400 scans.
Lets start by looking
at a 13C spectrum of diethyl phthalate obtained by the
FT technique;
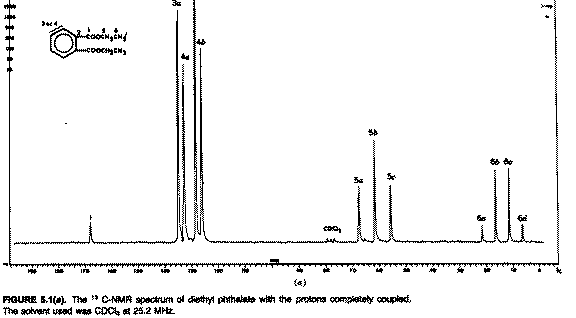
First we
note the wide chemical shift range of the signals. Note also that the
signal we attribute to the methyl group is approximately a 1:3:3:1 quartet,
the methylene approximately a 1:2:1 triplet, and the aromatic CHs
approximately 1:1 doublets. The intensity ratios suggest this is due to
coupling and the multiplicities that it is due specifically to coupling of
the spin 1/2 13C with the spin 1/2 protons and nothing
else (ie the 2nI+1 rule, I being the spin number). Before we move on to
discuss how this coupling may be useful to us, let us remind ourselves of how
the coupling arises. Remember the energy level diagram of one spin 1/2
nucleus in a magnetic field;
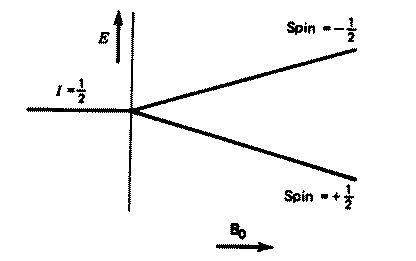
The precessing magnetisation vector either reinforces or opposes
Bo, so that locally at least, other nuclei will perceive two slightly
different values of Bo. Since the populations of each energy level are
practically identical (equation 5), another nucleus close-by will resonate
with equal probability at two slightly different Larmor frequencies (equation
1). The difference between these two frequencies is what we know at the
coupling constant J. Its value depends on how the perturbation in Bo
is transmitted between the two nuclei, and this is normally achieved
via the intervening electrons (hence the term through bond coupling).
When two identical nuclei are involved, three slightly different and equally
spaced values of Bo, with the middle one being twice as probable as
the highest or lowest, hence the 1:2:1 triplet coupling pattern we are
familiar with. In spin terms, we say that four configurations are possible;
+1/2,+1/2; +1/2, -1/2; -1/2, +1/2; -1/2, -1/2. As the middle two are of equal
energy, this manifests as a double height peak, ie a 1:2:1 triplet.
If we remember that J(coupling) = x  o/106,
these visible in the carbon spectrum above look in the
range JC-H ~ 6 x 22 ~ 130 Hz. Notice that carbon appears not to couple
with other carbons, only with protons in the same molecule. This is because
the probability that two 13C-13C
nuclei will be close enough to couple is 100 times less than the probability
of finding 13C-12C as adjacent
nuclei.
o/106,
these visible in the carbon spectrum above look in the
range JC-H ~ 6 x 22 ~ 130 Hz. Notice that carbon appears not to couple
with other carbons, only with protons in the same molecule. This is because
the probability that two 13C-13C
nuclei will be close enough to couple is 100 times less than the probability
of finding 13C-12C as adjacent
nuclei. Now look at the next spectrum;
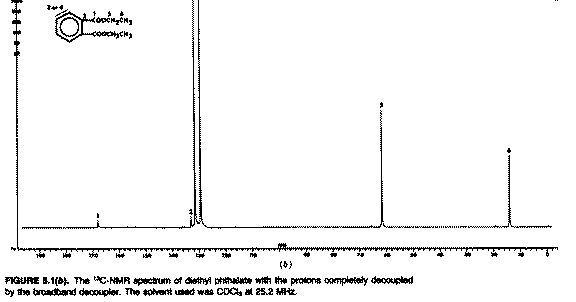
There are seven singlets; the spectrum is certainly
less cluttered because the coupling has gone! It has been removed by a
technique called broad band 1H decoupling. During
measurement of the 13C FID, the entire proton resonance
region of the compound (ie from about -2 to + 10 ppm) was irradiated with
"white noise" radio frequency. This has the effect of increasing the rate of
transition between the.proton low and high energy spin states such that only
one average magnetic field is experienced by any individual carbon nucleus,
and the manifestation of coupling vanishes. As far as the carbon is concerned
all the protons cease to exist (well, almost. There is an effect called the
nuclear Overhauser effect or nOe which does very odd things to the
intensity of the carbon line, normally increasing it by a factor of two
or more and hence is one of the factors making integration of
13C spectra unreliable. More of which in other lecture
courses!). Normally for 13C both decoupled and coupled
spectra are recorded, but the latter with a small modification called
"off-resonance proton decoupling";
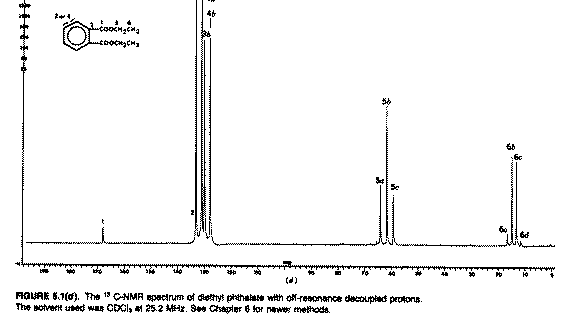
Here the proton decoupler is actually switched on during
13C measurement but its frequency is centred at about
-10 ppm. It has the effect of removing any coupling between
13C and 1H that occurs through more
than one bond (H-C-C and longer range couplings of < 10Hz)
but leaving coupling due to directly bonded atoms (ie H-C of >125
Hz) still visible (above). This reduces the complexity of the spectrum and
offers a significant gain in the intensity of the signals (~ four fold
reduction in measurement time) from the nOe enhancement referred to above.
Together, these two spectra give the following information;
- The
proton decoupled spectrum tells the number of unique types of carbon atoms in
the molecule (ie a mono-substituted pheny group has four unique carbon
atoms)
- The off-resonance spectrum tells how many hydrogen atoms are
attached to each unique carbon (quartet=3, triplet=2, doublet=1,
singlet=none).
- From the chemical shift of each carbon, much information
about the environment of the carbon can be gleaned.
The typical
chemical shift ranges of carbon nuclei are as follows;
| C (alkane) | ~ 0 - 30 ppm
|
| C (alkene) | ~ 110 - 150 ppm
|
| C-N | ~ 50
|
| C-O | ~ 60
|
| C-F | ~ 70 ppm.
|
| Aromatic | ~ 110 - 160 ppm
|
| Ester, amide, acid, | ~ 160-170 ppm
|
| Ketone, aldehyde | ~ 200-220 ppm.
|
You should be able to use this information to
deduce typical functional groups present in molecules. One or more examples
of this will be presented in the lecture course.
Return to Index page
Copyright (c) H. S. Rzepa and ICSTM Chemistry Department, 1994, 1995.



 o/106,
these visible in the carbon spectrum above look in the
range JC-H ~ 6 x 22 ~ 130 Hz. Notice that carbon appears not to couple
with other carbons, only with protons in the same molecule. This is because
the probability that two 13C-13C
nuclei will be close enough to couple is 100 times less than the probability
of finding 13C-12C as adjacent
nuclei.
o/106,
these visible in the carbon spectrum above look in the
range JC-H ~ 6 x 22 ~ 130 Hz. Notice that carbon appears not to couple
with other carbons, only with protons in the same molecule. This is because
the probability that two 13C-13C
nuclei will be close enough to couple is 100 times less than the probability
of finding 13C-12C as adjacent
nuclei.
