

1. The multistage synthesis consists of a sequence of at least three reactions which, as with the earlier Techniques experiments, will illustrate a particularly important or interesting transformation. A major objective is to develop the skills necessary for tackling a multistep synthesis.
2. Normally, no schedule will be issued, but a leading reference will be given. You are required to work from the literature information and adapt it, if necessary, to your process.
3. Although superficially similar to the second year 'projects', these syntheses are more demanding of skill and expertise. Some of the reactions require particular attention to detail and precise control of the reaction conditions. Take very careful account of the reported procedure and think carefully about what you are trying to achieve in every operation and be prepared to adjust the technique to fit in with your particular available equipment and materials.
4. Pay particular attention to work-up procedures. In many respects, it is skilful and thoughtful work-up technique that is the key to successful chemical synthesis.
5. Spectral and other appropriate physical/analytical data should be collected on all isolated compounds. This data must be interpreted to the highest possible degree in order to properly characterise your products.
6. The target molecules are all in the literature. When you have found a suitable synthetic sequence, a brief proposal covering the reactions involved and a hazard assessment of the materials and techniques must be submitted to a Staff Demonstrator or Dr. Rzepa or Dr. Marsden for approval and authorisation to start the project. Unless there is a particular contraindication, (e.g. high cost or hazard) aim to make ~ 1 g of product.
7. As these reactions are unscheduled, the hazard assessment must be entered into your COSHH Book before approval will be given. Guidance for COSHH assessments will be given by the College Safety Director on a date to be advised. No responsibility is accepted for any use of these instructions other than at Imperial College Chemistry Department.
8. The total synthesis should be written up as a single document in the style of a paper for J. Chem. Soc., Perkin Trans. I and handed in together with the product samples.
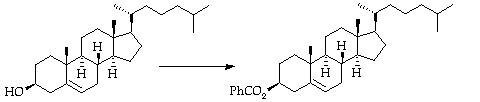
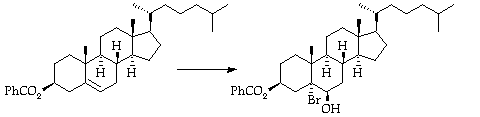
Stage 2: Bromohydrin formation
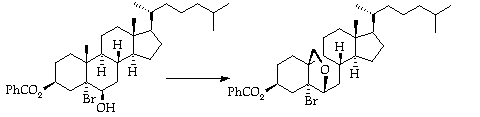
Stage 3: Lead Tetracetate/Iodine Remote Oxidation
References
1. M.C. Alpoim, PhD Thesis, Imperial College, 1980 and references there cited.
Stage 2: Tungsten hexachloride homocoupling.
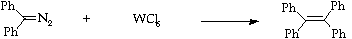
Stage 3: Cyclopropanation
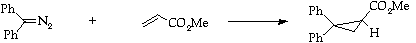
References.
1. T.G. Back, D.H.R. Barton, M.R. Britten-kelly and F.S. Guziec Jnr., J. Chem. Soc., Perkin Trans. I, 1976, 2079 and references there cited.
2. E. Hardegger, Z. El Heweihi and F.G. Robinet, Helv. Chim. Acta, 1948, 31, 439.
3. J.D. Roberts and C.M. Regan, Analyt. Chem., 1952, 24, 360.
4. W.M. Jones, T.H. Glenn and D.G. Baarda, J. Org. Chem., 1963, 28, 2887.
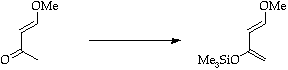
Stage 2: Alkyne cycloaddition
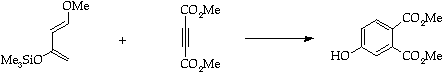
Stage 3: Alkene cycloaddition.
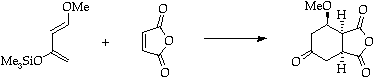
References
1. S. Danishefsky and T. Kitahara, J. Am. Chem. Soc., 1974, 96, 7807.
2. P. Cazeau, F. Duboudin, F. Moulines, O. Babot and J. Dunogues, Tetrahedron, 1987, 43, 2089.
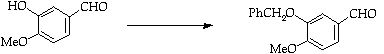
Stage 2: Aldehyde condensation
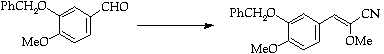
Stage 3: Amination
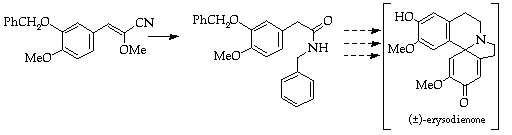
References
1. R.D. Bracho, DIC Thesis, Imperial College, 1974.
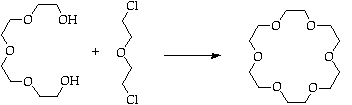
Purify via MeNO2 complex
Stage 2: Synthesis of 1-bromobenzocyclobutene
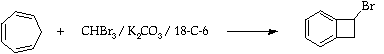
Stage 3: Alkylation of triphenylphosphine
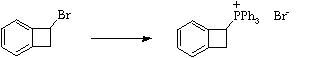
References
1. G. Johns, C.J. Ranson and C.B. Reece, Synthesis, 1976, 515.
2. M.R. DeCamp and L.A. Viscogliosi, J. Org. Chem., 1981, 46, 3918.
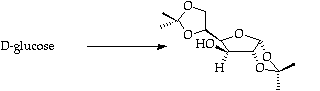
Stage 2: Xanthate formation
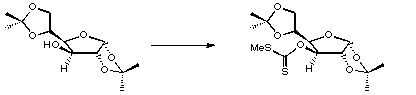
Stage 3: Deoxygenation
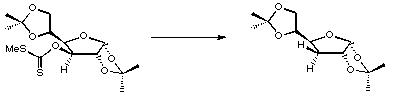
References
1. D.H.R. Barton and S.W. McCombie, J. Chem. Soc., Perkin Trans. I, 1975, 1574.
2. For a general account, see, O.T. Schmidt in 'Methods in Carbohydrate Chemistry', Eds. R.L. Whistler and M.L. Wolfrom, Academic Press, New York, 1963, vol.2, p. 320.
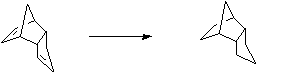
Stage 2: Lewis acid catalysed equilibration
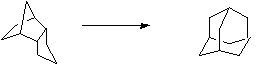
Stage 3: Oxidation
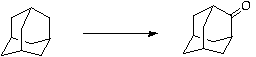
References
1. P. von R. Schleyer, M.M. Donaldson, R.D. Nicholas and C. Cupas, Org. Synth., 1962, 42, 8.
2. H.W. Geluk and J.L.M.A. Schlatman, Tetrahedron, 1968, 24, 5361.
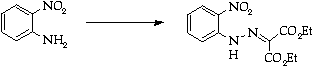
Stage 2: Aminotriazole synthesis
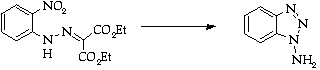
Stage 3: Benzyne trapping
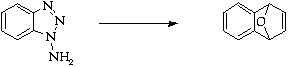
References
1. C.D. Campbell and C.W. Rees, J. Chem. Soc. (C), 1969,742.
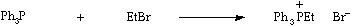
Stage 2: Simple coupling
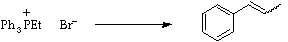
Stage 3: Double coupling
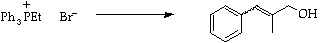
Discuss the geometry of the products
References
1. M. Schlosser, G. Müller and K.F. Christmann, Angew. Chem. Int. Ed. Engl., 1970, 5, 667.
2. E.J. Corey and H. Yamamoto, J. Am. Chem. Soc., 1970, 92, 226.
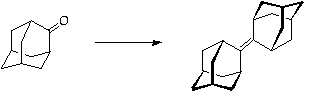
This step is potentially dangerous and requires very careful technique. Please discuss the method and safety precautions with DAW or HSR before commencing.
Stage 2: Epoxidation
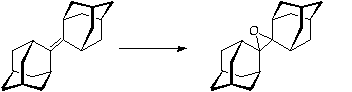
Stage 3: Rearrangement
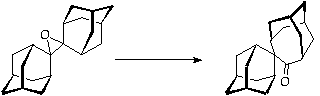
References
1. J.E. McMurray and M.P. Fleming, J. Org. Chem., 1976, 41, 896.
2. H. Wynberg, E. Boelema, J.H. Wierniga and J. Strating, Tetrahedron Lett., 1971, 181.
3. N.N. Schwartz and J.H. Blumbergs, J. Org. Chem., 1964, 29, 1976.
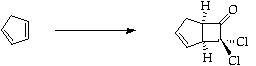
Stage 2: Ring opening
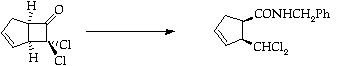
Stage 3: Ring expansion
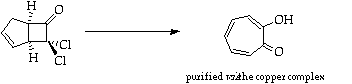
References
1. L. Ghosez, R. Montaigne, A. Roussell, H. Vanlierde and P. Mollet, Tetrahedron, 1971, 27, 615.
2. H.C. Stevens, D.A. Reich, D.R. Brandt, K.R. fountain and E.J. Gaugan, J. Am. Chem. Soc., 1965, 87, 5257.
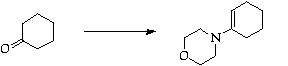
Stage 2: Robinson annulation
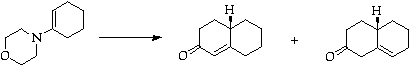
Separate by flash chromatography
Stage 3: Copper catalysed Michael addition
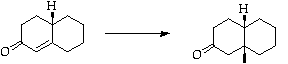
References
1. S. Hunig, E. Lücke and W. Brenninger, Org. Synth., 1961, 41, 65.
2. R.L. Augustine and J.A. Caputo, ibid., 1965, 45, 80.
3. G.H. Posner, Org. React., 1972, 19, 80.
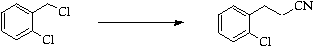
Stage 2: Cyclisation
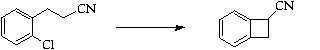
Stage 3: Thermal ring opening and cycloaddition
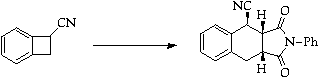
References
1. P. Radlick and L.R. Brown, J. Org. Chem., 1973, 38, 3412.
2. R. Bonnet and J. Skorcz, J. Med. Chem., 1966, 9, 656.
3. D.W. Jones and G. Kneen, J. Chem. Soc., Perkin Trans. I, 1976, 1647.
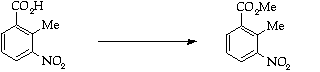
Stage 2: Batcho - Leimgruber indole synthesis, Step 1
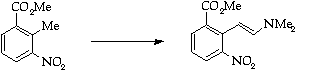
Stage 3: Batcho - Leimgruber indole synthesis, Step 2
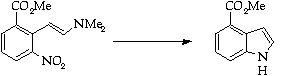
References
1. A.P. Kozikowski, H. Ishida and Y.-Y. Chen, J. Org. Chem., 1980, 45, 3350.
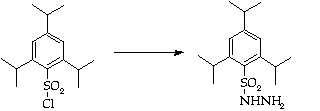
Stage 2: Hydrazone synthesis
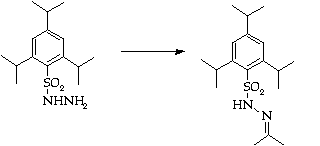
Stage 3: Regioslective allylation
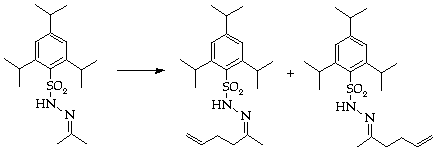
Assess isomeric ratio by nmr spectroscopy.
References
1. A.G.M. Barrett and R.M. Adlington, J. Chem. Soc., Perkin Trans. I, 1981, 2848.
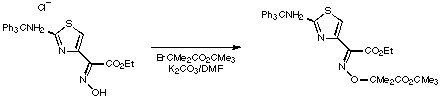
Stage 2: Ester hydrolysis.
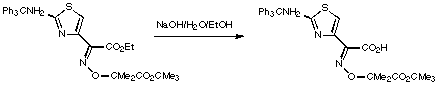
Stage 3: Coupling to the [[beta]]-lactam.
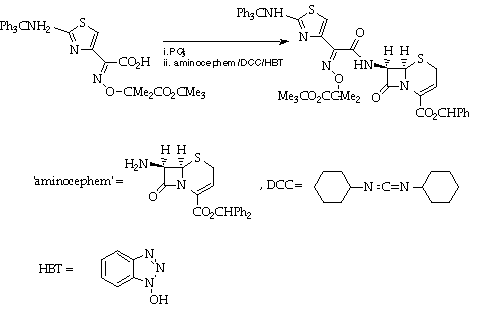
References
A Glaxo schedule will be provided.
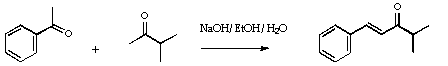
Stage 2: Complexation of the enone.
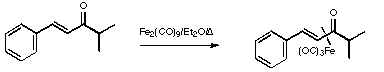
Stage 3: Synthesis of the vinylketene complex.
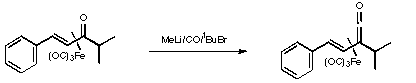
References
G. Morris, PhD Thesis, Imperial College, London, 1992.