Email Discussion: 69 (Poster) Differential solvation? Victor M. Rosas Garcia
URA 1679 du CNRS, Processus d'Activation Moléculaire,
24 rue Lhomond 75231 Paris Cedex 05, FRANCE
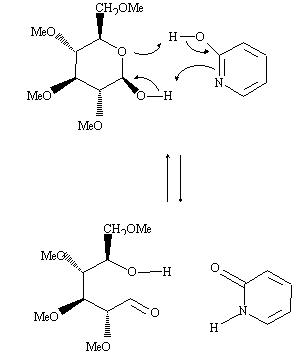
Bifunctional compounds such as 2-(1H)-Pyridone (1) have been reported to catalyze a variety of chemical reactions, for example mutarotation of glucose, hydrolysis of esters, aromatic nucleophilic substitutions. All these bifunctional catalysts are tautomeric compounds, usually weak acids and bases, which are able to exchange simultaneously two protons, being converted into their tautomeric form during the process of catalysis: from 2-hydroxypyridine to 2-pyridone in the example shown, the mutarotation of tetramethylglucopyranose (non protic solvent). The bifunctional nature of the catalyst allows a significant gain in entropy and the complexation insures a hydrophobic medium, which represent analogies with the mode of action of enzymes, giving rise to a large interest for the understanding of the whole process.

The role of the tautomeric equilibrium in the catalytic efficiency and in the recovering of the catalytic species is still not completely understood. We have devoted our first experiments in this area to the study of the tautomeric equilibrium itself, in particular the role of substituants on the stability of the tautomers and on the kinetics of exchange. For this purpose, we have synthesized two pyridines bearing a ester group or a amide group in position 3, which may give rise to intermolecular hydrogen bonds: 2-hydroxyl-3-(N-ethyl) carboxamido pyridine 1 and 2-hydroxy-3-carboxyethylpyridine 2.
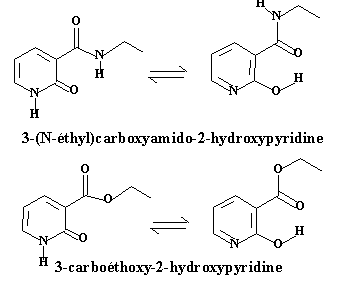
Because they do not tautomerize and are less prompt to dimerize, the N-methyl and O-methyl derivatives 1a, 1b, 2a and 2b were synthesized as references for spectroscopic studies: the two tautomeric forms, lactame (cyclic amide) and lactime (hydroxypyridine) could then be easily identified by 1H NMR and by UV spectrophotometry.
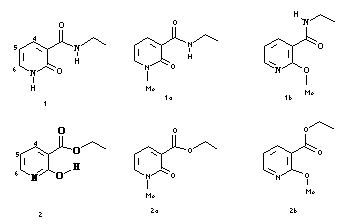
UV spectra of the N-methyl and O-methyl derivatives allow the assignment of absorbance maxima at 300 nm and 330 nm to the lactim and lactam chromophores respectively.
The effects of temperature and the addition of a polar solvent (methanol) on the UV spectra were separately studied for the amide 1 and the ester 2, for 2.10-3 mol.L-1 solutions in chloroform.
The UV spectrum of amide 1 showed essentially one maximum at 320 nm. Cooling (from 53°C to 12°C) or addition of MeOH caused very limited changes to the spectra, suggesting that only the lactam is present, and that its UV spectrum does not change significantly upon dimerization or complexation with MeOH.

for the Absorbance of Amide 1 (2.10-3 mol.L-1 in CHCl3).
On the UV spectrum of ester 2, two absorbance maxima appear. Each maximum is the sum of the absorbances of monomers and/or dimers for one tautomeric species. Upon cooling (from 57°C to 4°C), the lactam absorbance increases while the lactim absorbance decreases. Upon addition of MeOH, the lactim absorbance decreases and the lactam absorbance increases while (2lactam.MeOHn) complexes form.

for the Absorbance of Ester 2 (2.10-3 mol.L-1 in CHCl3).
These observations tend to establish the coexistence of both tautomers only for the ester, the amide existing mainly as a lactam. They are consistent with a differential stabilization of lactam in dimers and of lactim in monomers. The monomer-dimer equilibrium shifts with temperature (or concentration), which results in a shift of the protomeric equilibrium. As was previously suggested for other 3-carboxy-substituted 2-pyridones, the formation of an intramolecular hydrogen bond to the ester carbonyl stabilizes the lactim monomer.
We suggest that the relative stabilization of the lactam results from intramolecular-hydrogen bonding; an hydrogen-bond between the lactam carbonyl and the amide N-H seemingly forms despite the possibility of an hydrogen-bond between the lactim -OH and the amide carbonyl.
As expected, the intramolecular hydrogen-bond involving the amide N-H stabilizes the lactam whereas the ester carbonyl stabilizes the lactim.
The 1H NMR spectra, aromatic and protomeric protons, of 1 and 2 were observed upon varying concentration or temperature.
At high concentrations (1 mol.L-1) the aromatic signals of ester 2 almost match that of its N-methyl derivative, and shift toward that of its O-methyl derivative upon dilution. The NMR spectrum of 2 at -60°C and 0.1 .10-3 mol.L-1 is essentially similar to that recorded at 23°C and 10 .10-3 mol.L-1. These results support the hypothesis of a monomer-dimer equilibrium with lactam rich dimers and lactim rich monomers. Dilution causes dissociation of the dimers and therefore a shift in the protomeric equilibrium toward the lactim tautomer. However, the NMR data do not fit the binding isotherm calculated for an equilibrium involving a single (lactim) monomeric species and a single (lactam) dimeric species. We suggest that lactim monomers equilibrate with lactim dimers, which themselves equilibrate with lactam dimers. Accordingly, the protomeric proton of ester 2 shifts upon concentration: downfield (formation of lactim dimers) and then slightly upfield (formation of lactam dimers).
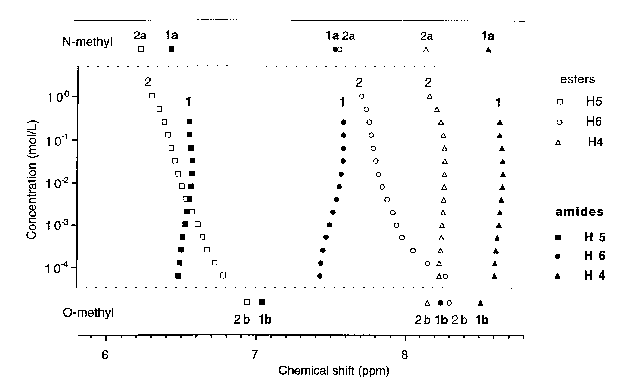
for Amide 1 and Ester 2 at Different Concentrations.
References: Above, O-Methyl Derivatives, Below: N-Methyl Derivatives.
At no concentration the NMR spectrum of 1 matches that of its O-methyl derivative. Dilution causes a shift of H5 and H6 signals, upfield (downfield for ester 2), and of the protomeric proton. The observed data fit a binding isotherm calculated for a simple dimerization, which results in a sigmoidal curve when the concentration is represented on a logarithmic scale. The best fit was obtained for a dimerization constant of 280 mol.L-1 (estimated error margin ±10%).
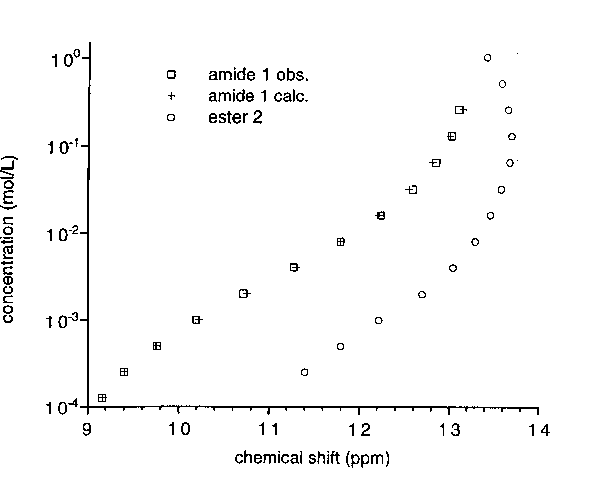
for Amide 1 and Ester 2. For Amide, Data Calculated for a simple Dimerization Isotherm with a Dimerization Constant of 280 mol-1.L.
NMR results are consistent with UV data and support the absence of lactim tautomer for 1 and a simple equilibrium between lactam monomers and dimers, with the formation of an intramolecular hydrogen-bond between the lactam carbonyl and the amide N-H: (i) the chemical shift of the amide N-H is relatively high and concentration independent; (ii) the unshielding effect of the carbonyl in position 3 on H4 is stronger for amide 1 than for ester 2.
Our aim was to correlate the tautomeric properties of substituted pyridones to their efficiency as bifunctional catalysts. We chose as a model reaction the aromatic nucleophilic substitution of fluoronitrobenzenes by substituted piperidines (2], [3), the conditions (concentrations of reactives) being chosen in order to follow the kinetics by measuring the absorbance of the product.
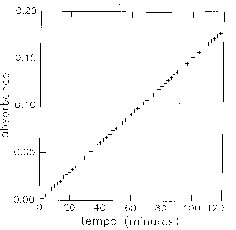
For the substitution of 2-fluoro-5-nitrobenzonitrile by piperidine (in excess) at 25°C in CHCl3, the variation of absorbance with time is characteristic of a first order kinetics law:
v = k [FB], with k = kapp [Pip],
with FB: 2-fluoro-5-nitrobenzonitrile and Pip: Piperidine.
The measured parameter is k; kapp is calculated.
For concentrations up to 5.10-3 mol.L-1, the results are consistent with the law: kapp = (k1k2/k-1) + (k1k3/k-1) [Cat] (Cat: catalyst).
By varying the concentration of catalyst, (k1k3/k-1) can be measured (slope of the curve). In order to compare our results with literature data, we calculated the ratio q = k3/k2, the common criteria for catalytic efficiency being a value of at least 50.

5.10-3 mol.L-1), for Solutions of 2-Fluoro-5-nitrobenzonitrile 9.63.10-5 mol.L-1, Piperidine 1.639.10-3 mol.L-1 in CHCl3 at 25°C.
The substitution in position 3, though non inhibiting catalytic efficiency, seems to diminish it rather largely, the effect being more serious for the ester:
Catalyst kCat = k1k3/k-1 q = k3/k2 Pyridone 1.39 267 Amide 0.418 80 Ester 0.359 69
kCat in mol-2.L2.s-1, k2 = 5.20.10-3 mol-1.L.s-1
For higher concentrations, the variation of k1k3/k-1 is no longer linear. Though, when one considers the concentration in monomer instead of the total concentration in catalyst, the variation becomes linear again. It can be fitted for instance for pyridone itself by taking into account the dimerization equilibrium, with a dimerization constant of 2000. For such a dimerization constant, the dimeric form becomes predominant (dimer/monomer > 1) for a total concentration of pyridone higher than ca 10-3 mol.L-1, which corresponds on the curve to a significant slowering of catalytic efficiency.
These observations suggest that, at higher concentrations in catalyst, the process gets slowed down because of a higher proportion of dimer, the catalytic species remaining the monomeric form.
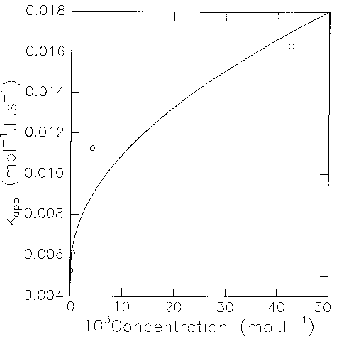
5.10-2 mol.L-1), for Solutions of 2-Fluoro-5-nitrobenzonitrile 9.63.10-5 mol.L-1, Piperidine 1.639.10-3 mol.L-1 in CHCl3 at 25°C.
The better efficiency of the amide, which always exists mainly as a lactim tautomer (monomeric then dimeric while concentration is getting higher), suggests a catalytic cycle initiated by the complexation of the intermediate with the lactim form of the catalyst.
The observed spectrophotometric and 1H RMN properties of amide 1 and ester 2 show that these derivatives exhibit considerable differences in their tautomeric behaviors in CHCl3. Intramolecular hydrogen bonds stabilize the lactam form of 1 (amide N-H bond involved) and the lactim form of 2 (ester carbonyl group involved), with dimerization influencing the tautomeric equilibrium. Because of its better hydrogen bonding ability, the lactam (better hydrogen bond donor and acceptor) is more abundant in dimers while the less polar lactim form predominates in monomers. It can be admitted that the proton exchange involved in the tautomery occurs within dimers. In polar or protic media, where the lactam predominates as a solvated monomer, the tautomery is more likely to occur between monomers and solvent.
Thus, the behavior of 2-pyridones in solution is the result of a number of equilibria: interconversion of tautomers, dimerization and solvatation.
How these equilibria affect the catalytic efficiency of both compounds, amide and ester, in aromatic nucleophilic substitution is at present not fully understood. Nevertheless, our preliminary results suggest the intervention of a lactim monomeric form in a non polar solvent. The next step will be to measure activation energies for the tautomeric equilibria by studying the effect of temperature on 1H RMN behavior of 1 and 2.
(1) Gallant, M; Viet, M.T.P.; Wuest, J. D. J. Am. Chem. Soc. 1991, 113, 721
(2) Pietra, F. Tetrahedron Lett. 1965, 6, 745
(3) Akpojivi, R. E.; Emokpae, T. A.; Hirst, J. J. Chem. Soc. Perkin Trans 2 1994, 443