
| Galactosyl donor | Product | Aspergillus orycae1 | Penicillium simplicissimum2 | Almond Meal |
|---|---|---|---|---|
| lactose | b-D-Gal(1-6)a-D-Glc-OMe 4 | 20 % | 27 % | 17 % |
| 4-nitrophenyl- b-D-galactopyranoside | b-D-Gal(1-6)-a-D-Glc-OMe4 total5 | 25 % 54 % |
25 % 56 % | 12 % 12 % |
| 2,4-dinitrophenyl- b-D-galactopyranoside | b-D-Gal(1-6)-a-D-Glc-OMe4 total5 | 32 % 62 % | 28 % 57 % | 20
% 21 % |
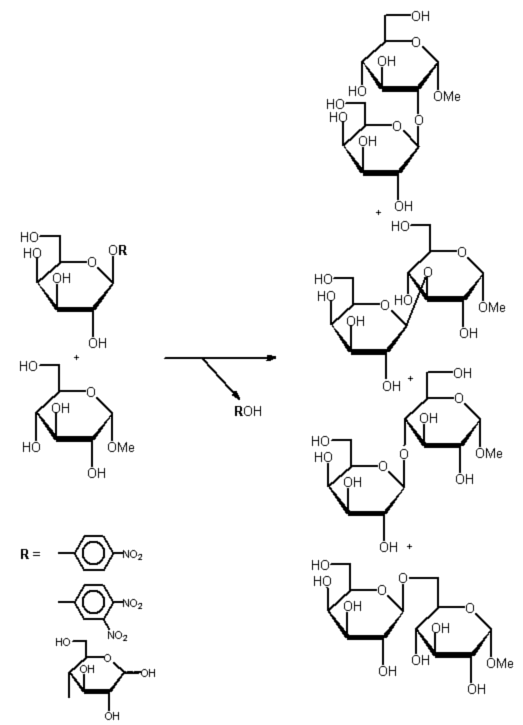
Almond meal almost exclusively catalysed the synthesis of b-D-Gal(1-6)a-D-Glc-OMe, whereas both fungal galactosidases gave a reasonable proportion of disaccharides involving secondary hydroxyl groups (all possible regioisomeres could be isolated, Fig. 1).
| Scheme 1 |
 |
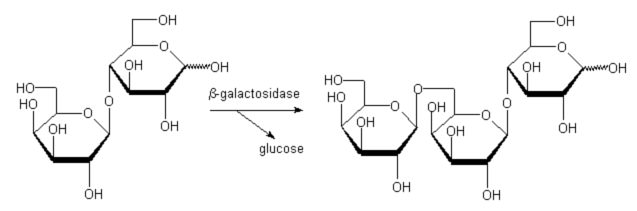
Employing lactose as donor we could isolate b-D-Gal(1-6)-b-D-Gal(1-4)-D-Glc as a side product (lactose serves as donor as well as acceptor, Fig. 2).
| Scheme 2 |
 |
The donor 2,4-dinitrophenyl b-D-galactopyranoside 5 gave the best yields in all investigated cases. 2,4-Dinitrophenyl 2-deoxy-2-fluoro-b-D-glucopyranoside has been found to be a mechanism-based inhibitor 6, and - to the best of our knowledge - 2,4-dinitrophenyl b-D-glycosides have never been used as donors in glycosidase-catalysed transglycosylation reactions so far.
As kinetically controlled reactions allow the use of crude enzyme preparations or culture filtrates as catalysts, we employed almond meal on the one hand and a crude culture filtrate of Penicillium simplicissimum on the other hand. In the latter case a novel b-galactosidase with high transglycosylation activity was detected.
Breeding conditions:
2,5% corn cobs, 1,2% carob seeds, 3% Solulyst A St, 0,5% NH4NO3 and 0,25% KH2PO4.
The b-galactosidase activity of the culture filtrate used was about 4 u/ml.
Attempts to increase the b-galactosidase activity were not successful so far.
Enzymatic reactions:
Conditions:
donor (0,2 mol/l), methyl a-D-glucopyranoside (1,5 mol/l), phosphate buffer (50
mmol/l), pH=5, 20° C
The course of reactions was followed by means of HPLC and stopped at maximum yield by heating to 90 °C for 10 minutes.
Separation of the transglycosylation products:
The isolation of all products was possible by using a charcoal-Celite column, which was prepared as follows: equal parts by weight of dry charcoal and Celite were slurried in water and packed into a glass column (50cm x 3,5cm). After denaturation of the enzyme the reaction mixture was applied to the column and eluted with a linear gradient from 0 to 30%(v/v) EtOH in water. The fractions containing transglycosylation products were collected, concentrated and rechromatographed on the same column (elution with a gradient from 6,5 to 10%(v/v) EtOH in water).