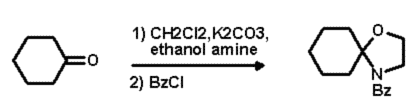
 This step was easily carried out in a one pot, two step synthesis (Scheme 2).
After a simple work up procedure, the crude residue was chromatographed to
produce the desired substrate which was then ready for the next step.
This step was easily carried out in a one pot, two step synthesis (Scheme 2).
After a simple work up procedure, the crude residue was chromatographed to
produce the desired substrate which was then ready for the next step.


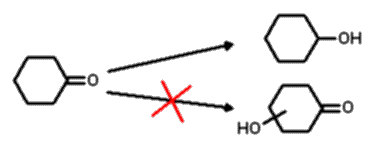 The problems of employing unprotected cyclic ketones as substrates for
hydroxylation with microorganisms is illustrated in the following example. Using
the well known fungus Beauveria bassiana ATCC 7159, cyclohexanone affords
cyclohexanol and not the desired hydroxylated ketone (Scheme 1).
The problems of employing unprotected cyclic ketones as substrates for
hydroxylation with microorganisms is illustrated in the following example. Using
the well known fungus Beauveria bassiana ATCC 7159, cyclohexanone affords
cyclohexanol and not the desired hydroxylated ketone (Scheme 1).
This stumbling block has been overcome by using the concept of reversible protecting / anchor groups. These protecting groups have been found not only prevent undesired side reactions, but also ease detection and aid docking into the active site. In particular, for ketones, results employing N-benzoylspirooxazolidines as anchor / protecting groups have been found to be very promising.
Step 1: Protecting / Anchor Group Introduction
 This step was easily carried out in a one pot, two step synthesis (Scheme 2).
After a simple work up procedure, the crude residue was chromatographed to
produce the desired substrate which was then ready for the next step.
This step was easily carried out in a one pot, two step synthesis (Scheme 2).
After a simple work up procedure, the crude residue was chromatographed to
produce the desired substrate which was then ready for the next step.
Step 2: Biohydroxylation Employing Beauveria Bassiana
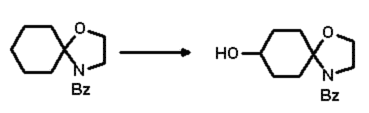 After dissolving the substrate in ethanol (100mg/ml), this was introduced (400mg
substrate/l culture) into the second stage of a standard two stage culture of
Beauveria bassiana ATCC 7159. After 48 hours, all of the spirooxazolidine
derivative was converted into a single, more polar compound. Extraction of the
culture with equal volumes of EtOAc (3 times) and chromatography of the residue
furnished the desired compound in good yield (Scheme 3, 49% isolated).
After dissolving the substrate in ethanol (100mg/ml), this was introduced (400mg
substrate/l culture) into the second stage of a standard two stage culture of
Beauveria bassiana ATCC 7159. After 48 hours, all of the spirooxazolidine
derivative was converted into a single, more polar compound. Extraction of the
culture with equal volumes of EtOAc (3 times) and chromatography of the residue
furnished the desired compound in good yield (Scheme 3, 49% isolated).
Step 3: Anchor / Protecting Group Cleavage
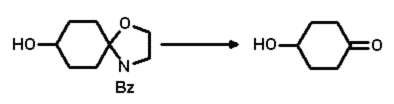 This last step was easily realised by simply dissolving the hydroxylated compound
in acetonitrile and adding acidic ion exchange resin (IR 120) at room temperature
with vigorous stirring (Scheme 4). After about 24 hours the cleavage was complete
and the hydroxylated ketone could be isolated in very good yield (83% isolated)
with chromatography.
This last step was easily realised by simply dissolving the hydroxylated compound
in acetonitrile and adding acidic ion exchange resin (IR 120) at room temperature
with vigorous stirring (Scheme 4). After about 24 hours the cleavage was complete
and the hydroxylated ketone could be isolated in very good yield (83% isolated)
with chromatography.