The Problem
N-Substituted pyrrolidin-3-ones (1) undergo enolisation to give the
C(4) enolate (3) preferentially.1
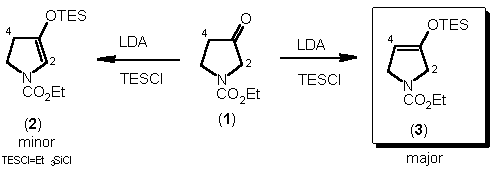
beta-Keto ester dianion as a C(2) enolate equivalent.2
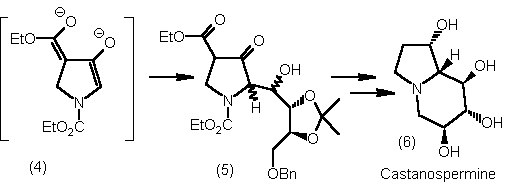
C(2)-enolates of pyrrolidin-3-ones have been generated from a beta-ketoester dianion (4) and this strategy
has been applied in the synthesis of polyhydroxy indolizidines (6).2
Aims
Study the enolisation of the pyrrolidin-3-one system
Direct enolisation towards C(2)
C-C bond forming reactions on the C(2) enolate using Mukaiyama aldol
conditions,3 fluoride ion-mediated4 and TMSOTF-catalysed
aldol-type reactions.5


