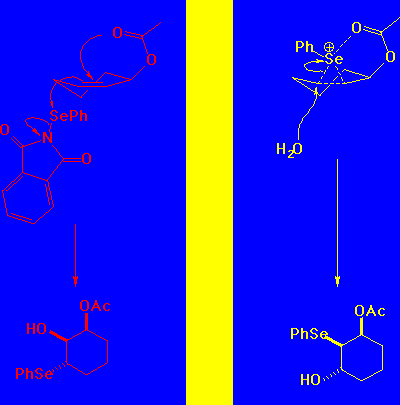
What's Going On in Hydroxyselenation of Acetoxycyclohexene?
J.B.Sweeney
School of Chemistry, Bristol University, Cantock's Close, Bristol UK BS8 1TS
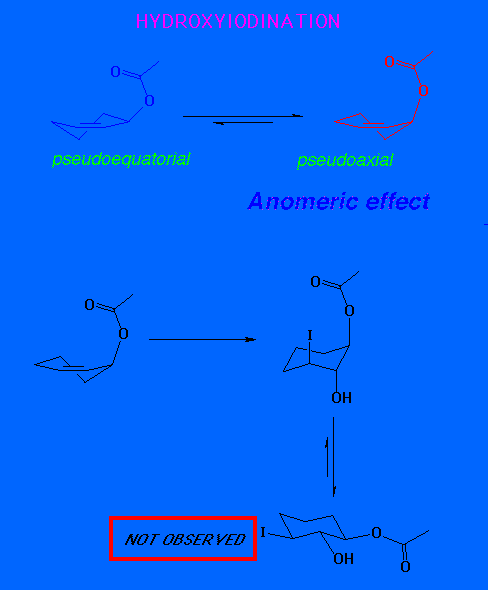
A pseudo axial conformation for the reactant would therefore give an all trans product.
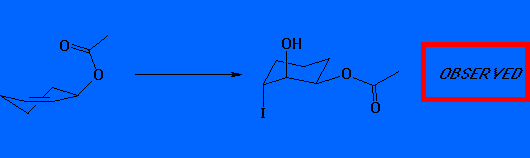
In fact, the observed product is the cis 2-hydroxy-3-acetoxy system. We conclude that the pesudo-equatorial conformer is therefore more reactive.
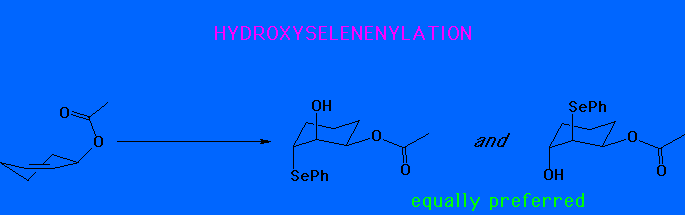
Once again, therefore the pseudo equatorial
conformer is more reactive. However, there is no face selectivity in
selenenylation.
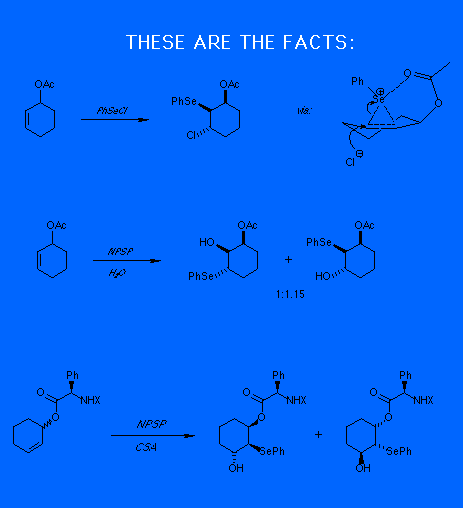
WHY?
We suggest there are
two reactions occuring, at roughly the same rate.