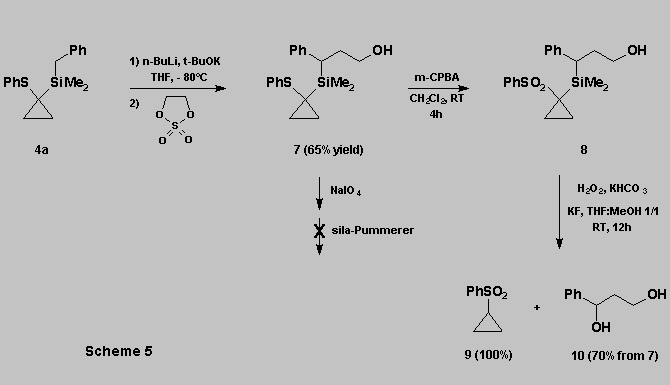
The oxidation can also be performed in the presence of a free
hydroxyl group. However :
* The free hydroxyl function
interfered with the course of the sila-Pummerer rearrangement.
* This can be circumvented using :
- the oxidation of the thioether
function to the corresponding sulfone 8 [11].
- the sulfone can then be subjected directly to the Tamao oxidation,
affording the desired diol 10 in good yield, along with the
cyclopropylsulfone 9 (Scheme 5).
This
approach provides an extension to the concept illustrated in scheme
1 and shows that oxidations of DMPTCS do not require the protection of
alcohol functions present on the substrate.

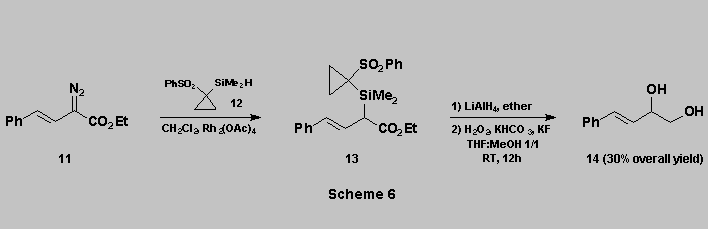
A further illustration that
phenylsulfonylcyclopropyl is an excellent nucleofugal group on silicon [12]
is provided by the sequence shown in Scheme 6.
The silane 12 was reacted with the alpha-diazoester
11 in the presence of Rh2(OAc)4 to give 13 [13]. Reduction of the ester function with LiAlH4 gave the unstable
ß-hydroxysilane which was directly oxidized using the same
conditions as above to afford the diol 14 in reasonable overall
yield.

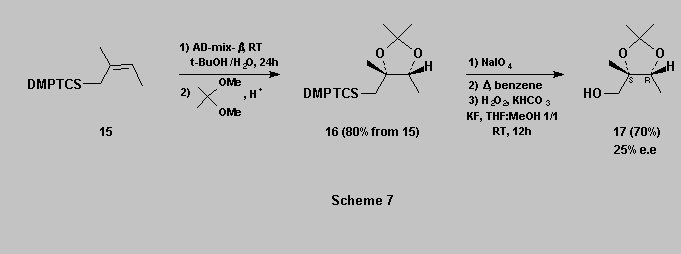
Polyhydroxylated silanes can also be obtained through Sharpless dihydroxylation [14]. For example, allylsilane 15 affords the desired diol which was purified as its acetonide 16. This latter is then oxidized as described above to afford the triol acetonide 17 [15] in 70% yield (Scheme 7).
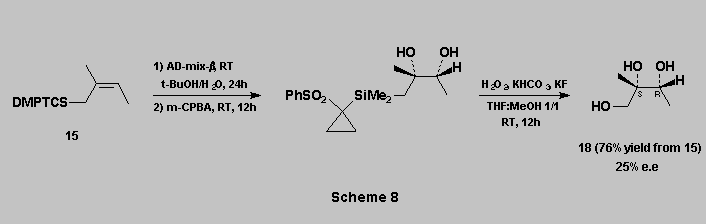

The C-Si bond oxidation can therefore be carried out equally well through the sulfoxide or the sulfone route, and in both cases in high yields.
