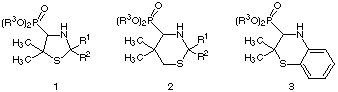
The α-aminophosphonic acids and their derivates, defined as phosphorus analogues of amino acids, are of interest because of their applications in pharmacological1-6 and agriculture7-9 areas.
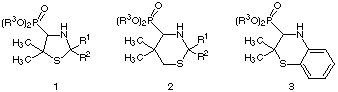
Scheme 1: Familiar α-aminophosphonic acid esters 1-3
In the course of our investigations on the synthesis of 4-thiazolidinyl phosphonates10 1 as intermediates of phosphonic acid analogues of penicillamine we became interested in the chemistry of the familiar six membered heterocyclic compounds.
In this paper we report the first synthesis of the novel racemic 1,3-thiazinan-4-yl-phosphonic acid esters 2 and 2-H-1,4-benzothiazin-3-phosphonic acid esters 3. One standard synthesis of α-aminophosphonic acids involves thermal addition of dialkyl phosphonates to imines11-14. By this way we decided to synthesize the desired compounds 2 and 3 starting from 2H-1,3-thiazines 4 and 2H-1,4-benzothiazines 5.
Results and Discussion
At the first time the 2H-1,3-thiazines 4 were prepared analogue to the modified Asinger-reaction, which is well-known for the preparation of 3-thiazolines15. Therefore, in a multi component one pot reaction 3-chloro-2,2-dimethylpropanal16 is treated with a further carbonyl compound in the presence of sodium hydrogen sulfide and aqueous ammonia as shown in scheme 2.
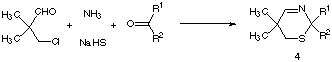
Scheme 2: One pot synthesis of 2H-1,3-thiazines 4
The 2H-1,4-benzothiazine 5 was prepared according to the procedure described by Shridhar17. The synthesized heterocyclic imines 4a-c and 5 are listed in table 1.
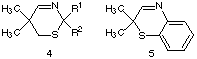
Table 1: 2H-1,3-thiazines 4 and 2H-1,3-benzothiazines 5
4,5 R1 R2 Yield [%]
4a -(CH2)4- 36
4b -(CH2)5- 37
5 -- -- 17
The
preparation of the new α-aminophosphonic acid esters follows the
synthesis of the analogue thiazolidin-4-yl-phosphonates described
earlier11,12. In practice, the synthesis is quite simple, requiring
refluxing of a mixture of the cyclic imines 4 and 5 with dialkyl
phosphite in ligroine for eighteen hours. After storage at -28[[ring]]C the
products crystallized in several days.
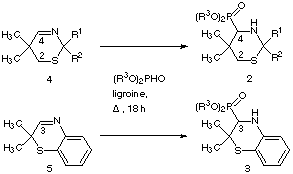
Table 2: Dialkyl phosphite adducts 2 and 3
2,3 R1 R2 R3 Yield [%] mp
[deg.C][a]
2a -(CH2)4- CH3 12 61
2b -(CH2)4- C2H5 31 76-78
2c -(CH2)5- CH3 47 78-79
2d -(CH2)5- C2H5 54 84
3a -- -- CH3 40 115-117
3b -- -- C2H5 74 110-111
[a]
uncorrected.
We also investigated the nature of the imine component on the rate of formation. According to their lower stability we obtained the phosphite adducts of 2H-1,3-thiazines in lower yields than 1,3-thiazolines and 2H-1,4-benzothiazines. Generally we received the diethylphosphite adducts with higher yields up to 74% than the dimethylphosphite derivates.
The 13C nmr spectra exhibit characteristic P-C signals seen as a doublet centered between 56.64 ppm and 60.66 ppm with coupling constants ranging from 147.7 Hz to 157.9 Hz. In addition with the observed 1H nmr data they are within the limits of usual values10,18-20 (table 3).
Table 3: 13C and 1H nmr data of 2 and 3
13C nmr data[a] 1H nmr data[a]
2,3 δ[C-P] 1J(C-P) δ[CH-P] 2J(H-C-P) 4J(H-C-C-C-P)
2a 59.64 156.8 3.14 23.6 9.1
2b 56.64 157.8 3.20 23.5 7.8
2c 59.86 156.8 2.96 23.9 8.9
2d 56.72 157.9 2.79 23.8 7.6
3a 60.14 148.4 3.89 12.1 --
3b 60.66 147.7 3.88 12.7 --
[a]
δ in ppm; J in Hz.
In contrast to recently synthesized 2H-1,3-thiazine derivatives with a carbon atom instead of the phosphorus atom 21, the proton spectra show an additional coupling of one of the diastereotopic methylene protons at C2. This may be explained by an unusual large long range, w-type coupling between 31P and 1H, which is well known for other systems22-28.
Acknowledgement: This research was supported, in part, by the Fonds der Chemischen Industrie and Degussa AG. The authors would also like to thank R. Irmer and S. Vieth for experimental assistance.
References and Notes:
(1) Stowasser, B., Budt, K.-H., Jian-Qi, L., Peyman, A., Ruppert, D.
Tetrahedron Lett. 1992, 33, 6625.
(2) Huber, J. W., Gilmore, W. F., Robertson, L. W. J. Med. Chem.
1975, 18, 106.
(3) Atherton, F. R., Hall, M. J., Hasall, C. H., Holmes, S. W.,
Lambert, R. W., Lloyd, W. J., Ringrose, P. S. Antimicrob. Agents
Chemother. 1980, 18, 897.
(4) Kametani, T., Kigasawa, K., Hiragi, M., Wakisaka, K., Haga, S.,
Sugi, H., Tanigawa, T., Suski, K., Fukawa, K., Irino, O., Saita,
O., Yamabe, S. Heterocycles 1981, 16, 1205.
(5) Atherton, F. R., Hall, M. J., Hasall, C. H., Lambert, R. W.,
Lloyd, W. J., Ringrose, P. S. Antimicrob. Agents Chemother. 1979,
15, 684.
(6) Allen, J. G., Atherton, F. R., Hall, M. J., Hasall, C. H.,
Holmes, S. W., Lambert, R. W., Nisbet, L. J., Ringrose, P. S.
Antimicrob. Agents Chemother. 1980, 18, 897.
(7) Emsley, J., Hall, D. The Chemistry of Phosphorous, Harper & Row,
London 1976, p. 494.
(8) Maier, L. Phosphorous, Sulfur and Silicon 1991, 61, 65.
(9) Schwerdtle, F., Bieringer, H., Finke, M. Z. Pflanzenkr.
Pflanzenschutz 1981, 9, 431.
(10) Martens, J., Kintscher, J., Lindner, K., Pohl, S., Saak, W.,
Haase, D. Liebigs Ann. Chem. 1991, 305.
(11) Schwarze, W., Drauz, K., Martens, J. Chem.-Ztg. 1987, 111, 149.
(12) Drauz, K., Koban, H. G., Martens, J., Schwarze, W. Liebigs. Ann.
Chem. 1985, 448.
(13) Gilmore, W. F., McBride, H. A. J. Am. Chem.Soc. 1972, 94, 4361.
(14) Takahashi, H., Yoshioka, Imai, N., Onimura, K., Kayashi, S.
Synthesis 1994, 763.
(15) Martens, J., Offermanns, H., Scherberich, P. Angew. Chem. 1981,
93, 680; Angew. Chem. Int. ed. Engl. 1981, 20, 668.
(16) Wilt, J. W., Aznavorian, P. M. J. Org. Chem. 1978, 43, 1285.
(17) Shridar, D. R., Reddy Sastry, C. V., Bansal, O. P., Pulla Rao, P.
Synthesis 1981, 913.
(18) Sum, V., Kee, T. P. J. Chem. Soc. Perkin Trans. I 1993, 2701.
(19) Hoppe, I., Schöllkopf, K., Nieger, M., Egert, E. Angew. Chem.
1985, 97, 1066; Angew. Chem. Int. Ed. Engl. 1985, 24, 1036.
(20) Hesse, M., Meier, H., Zeeh, B. Spektroskopische Methoden in der
organischen Chemie, 2. Auflage, Georg Thieme Verlag, Stuttgart,
New York 1984.
(21) Manikowski, J., unpublished results.
(22) Benezra, C. Tetrahedron Lett. 1969, 4471.
(23) Callot, H. J., Benezra, C. Can. J. Chem. 1970, 48, 3382.
(24) Griffin, C. E., Kundu, S. K. J. Org. Chem. 1969, 34, 1532.
(25) Cremer, S. E., Chorvat, R. J. J. Org. Chem. 1967, 32, 4066.
(26) Ross, J. A., Martz, M. D. J. Org. Chem. 1969, 34, 399.
(27) Nair, V., Young, D. A. Magn. Reson. Chem. 1987, 25, 937.
(28) Cohen, H., Benezra, C. Org. Magn. Reson. 1973, 5, 205.