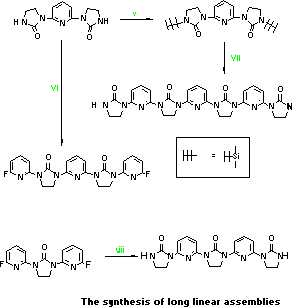
- IPI (2.60g),
dimethylthexylsilyl chloride (5.36g) and triethylamine 5.0g) in DMF
(50mL) were heated at 90 C under nitrogen overnight. The cooled
solution was diluted with ice-water (~200mL) and the
N,N'-bis-dimethylthexylsilyl-IPI [bis-dmt-IPI] collected (4.40g, 79%).
White crystals from ethyl acetate, m.p. 217-8 C. Similar derivatives
of PIPI (86%, m.p. 285-8 C) and IPIPIPI (85%, m.p. >340 C) both
recrystallised from toluene.
- IPI (2.47g, 0.01mol) and
2,6-difluoropyridine (2.79g, 0.024mol) in THF( 0mL) containing NaH
(1.20g, 50%, 0.025mol) were heated at reflux for 40h under nitrogen
and the poured into water to give FPIPIPF as white crystals from DMF,
m.p. 318-9 C.
- Bis-dmt-IPI (4.26g), t-Bu-IP and cesium fluoride
(0.2g) in DMF (100mL) were heated at 110 C for 2 days, cooled and
poured into water. The filtered product, bis-t-Bu-IPIPIPI (4.80g,
88%) produced white crystals from toluene m.p. >340 C. This product with
20% aqueous HCl at 80 C overnight gave IPIPIPI (91%), m.p. >340
C.
- PIP (8.28g, 0.03mol) and N-t-butylimidazolidinone
(9.37g,0.066mol) in THF containing NaH (3.20g, 50%, 0.067mol) were
refluxed for 48h under nitrogen. Most of the solvent was removed and
ice-water (~500mL) added to reprecipitate bis-t-Bu-IPIPI which
recrystallised from toluene (12.6g, 81%) m.p. 298-9 C. Heating this
compound (8.20g) in HCl (80mL, 20% aqueous) at 100 C overnight gave
IPIPI (6.31g, 98%) which recrystallised from aqueous TFA, m.p. >300
C.
Back to main page

