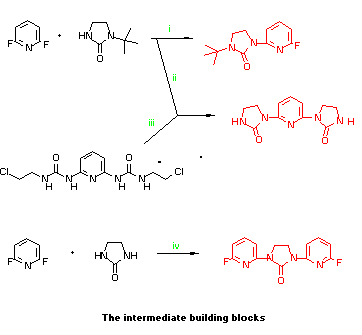
- N-t-Butylimidazolidinone (7.0g, 0.05mol) was added to THF (150mL)
containing NaH 2.64g, 50% in oil washed with hexane, 0.055mol) with gas
evolution. 2,6-Difluoropyridine (6.8g, 0.059mol) was added and the mixture
refluxed for 20h, most of the solvent removed and water (~500mL) added when
a solid precipitated. Recrystallisation from light petroleum 60-80 gave
2-fluoro-6-(N-t-butyl-imidazolidinon- N'-yl)pyridine as white crystals,
m.p.103-4 C.
- The reagents as in i (25.56g, 9.6g, 9.20g respectively) were refluxed
for 40h and worked up as above. Recrystallisation from ethyl acetate gave
2,6-bis-(N-t-butyl- imidazolidinon-N'-yl)pyridine (21.10g, 73.4%), m.p.
248-9 C. The same product could be obtained from 2,6-dichloropyridine and
the above reagents on DMF (100 C, 24h) in 60% yield together with
N-(2-chloro-6-pyridyl)-N'-t-butylimidazolidinone (34%).
The bis-t-butyl derivative (8.00g) and 20% aqueous HCl were refluxed
overnight and the mixture neutralised (4M NaOH) to give
2,6-bis-(imidazolidinon-N-yl)pyridine [IPI for short] (5.40g, 98%), m.p.
>340 C(DMSO).
- 2,6-Bis(chloroethylureido)pyridine (2.15g), NaH (0.96g, 50%) and THF
(50mL) were refluxed for 2 days, solvent removed and water added to give IPI
(1.30g, 78%).
- Imidazolidinone (4.48g, 0.05mol), THF (80mL) and NaH (5.28g, 50% in oil
washed as above, 0.11mol) and 2,6-difluoropyridine were mixed as above and
heated at reflux under nitrogen for20h. Water (~500mL) was added, and the
chilled solution filtered to give
N,N'-bis-(2-fluoropyridin-6-yl)imidazolidinone [PIP for short] (11.47g,
83%). Small amounts were recrystallised from ethyl acetate and large
quantities from DMF as white plates, m.p. 267-8 C (slowly changed to needles
on heating).
Back to main page

