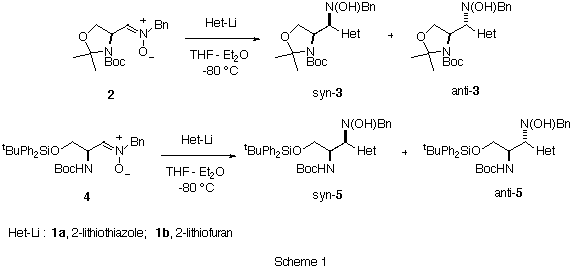
As a natural extension of these studies we have initiated a complementary investigation using a-amino nitrones as starting compounds.7 We wish to report herein a successful example of stereocontrol in nucleophilic additions of metalated heterocycles to a-amino nitrones by changing the protecting groups of the initial substrate. A proposal for explaining the stereochemical outcome of the reaction is also presented.

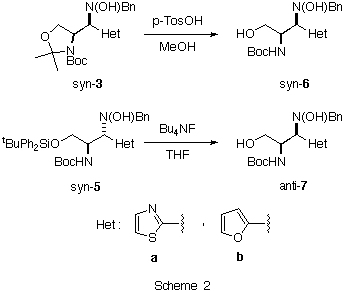
The reaction of 2-lithiothiazole 1a and 2-lithiofuran 1b with the nitrone8 2 in THF-Et2O as a solvent at -80 deg.C afforded syn-3a and syn-3b in good yields and high degrees of diastereoselectivity (Table 1, entries 1 and 2). On the other hand, the addition of the same metalated heterocycles 1 to the diferentially protected a-amino nitrone 4 gave in both cases anti-5a and anti-5b as major adducts (Table 1, entries 5 and 6). It is noteworthy that, contrary to the results obtained with a-alkoxy nitrones, the precomplexation of a-amino nitrone 2 with Lewis acids prior to the addition did not affect to the diastereoselectivity of the reaction (Table 1, entries 3 and 4). In the case of nitrone 4 a slightly inversion of the diastereoselectivity was obtained after precomplexation with diethyl aluminum chloride (Table1, entries 7 and 8).
Table 1. Additiona of Het-Li 1 to Nitrones 2 and 4. Entry Nitrone Het-Li Lewis Acid syn : antib yieldc (%) 1 2 1a none >= 95 : 5d 89 2 2 1b none >= 95 : 5d 92 3 2 1a Et2AlCl >= 95 : 5d 90 4 2 1b Et2AlCl >= 95 : 5d 88 5 4 1a none 12 : 88 87 6 4 1b none 10 : 90 90 7 4 1a Et2AlCl 60 : 40 86 8 4 1b Et2AlCl 59 : 41 89 a All reactions were carried out using 3.0 equiv. of the organometallic reagent. b Measured from the intensities of 1H NMR signals. c Determined on isolated mixtures of syn- and anti- adducts. d Only one compound was detected by NMR spectroscopy.
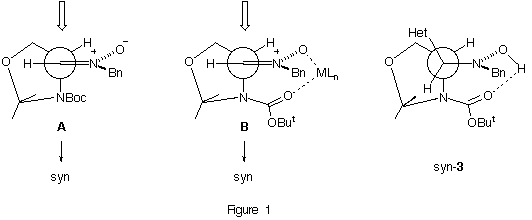
The stereochemical course of the addition reactions to nitrone 2 can be rationalized by invoking a transition state model A in a similar way to that proposed by us for the addition to the nitrone derived from D-glyceraldehyde.3b Model A is similar to that involved in nucleophilic additions to double bonds which has been proposed by Houk.10 In the case of the addition to nitrone 2 in the presence of a Lewis acid the no reversal of the stereoselectivity11 is consistent with a chelation model B (identical to A) in which a complexation between the nitrone oxygen and the carbamate oxygen is present.12 This proposal is supported by considering a transition state similar to the final products syn-3. It is possible to make such an assumption on the basis of the X-ray structures of syn-3a and syn-3b which present intramolecular hydrogen-bond interactions9 as shown in Figure 1 (the similarity of transition state model B and the final products syn-3 is evident).
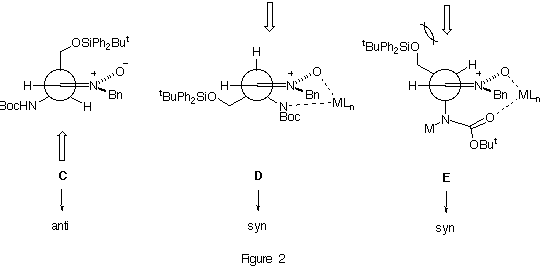
We propose for the addition reactions to the nitrone 4 a transition state model C in which the largest group is considered to be the silyloxymethyl group instead of the N-tert-butoxycarbonylamino one. Whereas molecular mechanics calculations13 gave a steric energy value of 15.86 kcal mol-1 for C, a higher value (18.08 kcal mol-1) was obtained when the N-tert-butoxycarbonylamino group was considered to be the largest one. The possibility of any intramolecular interaction like that sown in model D can be disregarded since no intramolecular hydrogen-bond interactions are observed in N-monoprotected a-amino nitrones by neither NMR techniques nor X-ray analysis.14 In the case of the addition to the nitrone 4 in the presence of diethyl aluminum chloride we assumed a transition state model E. This conformation can be stabilized by the complexation of the nitrone and carbamate moieties with the metal atom. The low values of diastereoselectivity observed can be explained by the unfavourable steric interactions between the silyloxy group and the incoming nucleophile.
In conclusion, we have achieved a stereocontrolled addition of metalated heterocycles to a-amino nitrones derived from L-serine by changing the protecting groups of the starting compound. The proposed transition state models can help to understand the course of the reaction and thus to extend the process to other a-amino nitrones derived from N,N-diprotected and N-monoprotected a-amino acids. These studies are actually undergoing in our laboratories and will be reported in due course.
References and Notes
1. (a) Duthaler, R.O. Tetrahedron 1994, 50, 1539 and references cited therein. (b) Enders, D.; Bettray, W.; Raabe, G.; Runsink, J. Synthesis 1994, 1322. (b) Shida, N.; Kabuto, C.; Niwa, T.; Ebata, T.; Yamamoto, Y. J. Org. Chem. 1994, 59, 4068. (c) Praly, J.-P.; Senni, O.; Faure, R.; Descotes, G. Tetrahedron 1995, 51, 1697. (d) Ward, R.S.; Pelter, A.; Goubert, D.; Pritchard, M.C. Tetrahedron: Asymm. 1995, 6, 93. (e) Dupradeau, F.-Y.; Hakomori, S.; Toyokuni, T. J. Chem. Soc. Chem. Commun. 1995, 221.
2. (a) Tennant, G. Imines, Nitrones, Nitriles and Isocyanides In Comprehensive Organic Chemistry. Barton, D.H.R.; Ollis, W.D. (Eds.) Pergamon Press. Oxford. 1979. Vol. 2. (b) Volkmann, R.A. Nucleophilic Additions to Imines and Imine Derivatives In Comprehensive Organic Synthesis. Trost, B.M.; Fleming, I. (Eds.) Pergamon Press. Oxford. 1991. Vol.1.
3. (a) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1992, 33, 4221. (b) Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1993, 34, 5475.(c) Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Chemistry. A European Journal 1995 (in press).
4. Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Synlett. 1993, 78.
5. Dondoni, A.; Merchan, F.L.; Merino, P.; Tejero, T. Synth. Commun. 1994, 24, 2551.
6. (a) Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1993, 34, 5475. (b) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synthesis 1994, 1450. (c) Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1994, 35, 9439.
7. Dondoni, A.; Merchan, F.L.; Merino, P.; Tejero, T.; Bertolasi, V. J. Chem. Soc. Chem. Commun. 1994, 1731.
8. Nitrones were prepared as described in Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino,P.; Tejero, T. Synth. Commun. 1994, 24, 2537.
9. Click here for ser1 and ser2 geometries.
10. Paddon-Row, M.N.; Rondan, N.G.; Houk, K.N. J. Am. Chem. Soc. 1982, 104, 7162.
11. It has been well-demonstrated by us that nucleophilic additions of metalated heterocycles to a-alkoxy nitrones can be stereocontrolled by carrying out the reaction in the absence or in the presence of Lewis acids to give syn- or anti-adducts, respectively (see refs. 1-4).
12. A very similar model has been proposed by Garner et al. for nucleophilic additions to the N-Boc L-serinal acetonide in the presence of aluminium. See: Garner, P.; Park, J.M.; Malecki, E. J. Org. Chem. 1988, 53, 4395.
13. Calculations were performed using Chem 3D Plus`.
14 (a) Merino, P.; Lanaspa, A.; Merchan, F.L.; Tejero, T. Acta Cryst. Sect. C 1995 (in press). (b) Merino, P.; Lanaspa, A.; Merchan, F.L.; Tejero, T. unpublished results.