One restriction using this enzyme is the requirement for dihydroxyacetone mono-phosphate in this reaction. Recently we have begun to investigate an aldolase (transketolase) that does not require phosphorylated substrates. The enzyme has been successfully over-expressed resulting in large quantities suitable for biotransformation (Hobbs et al., 1993).

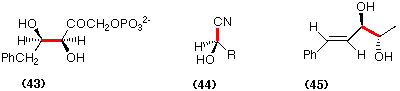
A less aesthetic process, but nevertheless a very useful transformation, involves the use of lyase enzymes which can catalyse the addition of HCN to aldehydes to give optically active cyanohydrins. Mandelonitrile lyase catalyses such an addition of HCN to a variety of aldehydes to afford the corresponding (R)-cyanohydrins (44) (Brussee et al., 1988). An enzyme that shows selectivity for the formation of (S)-cyanohydrins has been isolated.
Finally Fuganti et al.(1984) have explored the use of bakers' yeast for the controlled addition of acetaldehyde to ãß-unsaturated carbonyl compounds through an acyloin type of reaction. For example cinnamaldehyde is converted into 2(S),3(R)-5-phenylpent-4-ene-2,3-diol (45)in ca. 20% yield and in high diastereoisomeric excess.