To a solution of 1-ethinyladamantane (8.0 g, 50 mmol) in dry THF (120 ml), 50 mmol of butyl lithium (2.5 M solution in hexane, 20 ml) was added dropwise at room temperature. After stirring for 30 min, 3-oxo-camphorsulfonylimine ((3aS)-8,8-dimethyl-4,5,6,7-tetrahydro-3H-3a,6-methano-2,1-benzisothiazole 2,2-dioxide) [7] (4.9 g, 20 mmol) was added in five portions over a period of 10 min, and the mixture was refluxed for three days. After aqueous workup, extraction with dichloromethane and evaporation of the solvent, the crude product was purified by chromatography (silicagel/hexane:ethyl acetate 5:1). Yield: 8.0 g (73%).
Product C:
A solution of A (77 mg, 0.14 mmol) and PtCl2(PhCN)2 (7 mg, 0.015 mmol, 10 mol%) in 1 ml of chloroform was kept at 50 degree for three weeks. The unchanged platinum complex was precipitated by addition of hexane and filtered off. The resulting solution was evaporated to give an orange-brown solid (73 mg, 95%) which is pure enough for analytical purposes.
mass spectrum (EI, 70eV):
547 (M), 483 (M-SO2), 412 (M-adamantyl), 348 (M-SO2 -adamantyl),
135 (100%, adamantyl)
IR spectrum (KBr):
3432, 3316 (OH,NH), 2904, 2849 (CH), 2234 (acetylene)
optical rotation:
[alpha] (Na-D line, 24 deg.) = -4.1 (c=0.8 in EtOH)
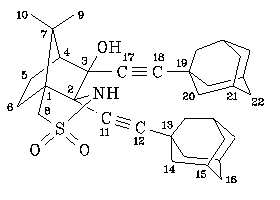
NMR spectra (for numbering of the atoms, see figure):

0.98 (s,3H,10-H), 1.43 (s,3H,9-H), 1.68 (m,13H,6-H+16-H+22-H), 1.78 (m,1H,5H), 1.91 ("s",6H,14-H or 20-H), 1.94 (m,7H,5-H+20-H or 14-H), 1.96 ("s",6H,15-H+21-H), 2.18 (m,1H,6-H), 2.00 (d, 4.7Hz,1H,4-H), 2.87 (s,1H,OH), 3.25 (s,2H,8-H), 4.99 (s,1H,NH).
13C-NMR (d-chloroform, 90.5 MHz):
23.6 (CH3,C-9), 23.8 (CH2,C-5), 24.1 (CH3,C-10), 27.82 (CH,C-15 or C-21), 27.84 (CH,C-21 or C-15), 28.3 (CH2,C-6), 29.6 (C,C-13 or C-19), 29.8 (C,C-19 or C-13), 36.28 (CH2,C-16 or C-22), 36.31 (CH2,C-22 or C-16), 42.56 (CH2,C-14 or C-20), 42.63 (CH2,C-20 or C-14), 48.4 (C,C-7), 51.3 (CH2,C-8), 56.9 (CH,C-4), 62.5 (C,C-1), 72.3 (C,C-2), 77.6 (C,C-11), 79.7 (C,C-17), 81.0 (C,C-3), 96.1 (C,C-12 or C-18), 98.3 (C,C-18 or C-12).
NMR spectra (for numbering of the atoms, see figure):
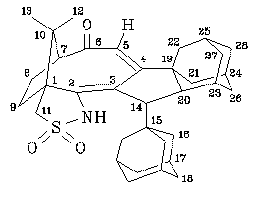
1.19 (s,3H,12-H or13-H), 1.23 (s,3H,13-H or 12-H), 1.30 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 1.36 (dm,11.3Hz,3H,16-H), 1.50 (m,1H,9-H or 8-H), 1.53 (m,6H,18-H), 1.55 (m,3H,18-H), 1.60 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 1.62 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 1.65 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 1.70 (m,2H,21-H or 22-H or 26-H or 27-H or 28-H), 1.72 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 1.73 (m,1H,9-H or 8-H), 1.75 (m,1H,20-H), 1.80 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 1.90 (m,1H,23-H or 24-H or 25-H), 1.93 (m,3H,17-H), 1.95 (m,1H,23-H or 24-H or 25-H), 1.98 (m,1H,14-H), 2.00 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 2.03 (m,1H,8-H or 9-H), 2.07 (m,1H,23-H or 24-H or 25-H), 2.20 (m,1H,8-H or 9-H), 2.58 (d,6.6Hz,1H,7-H), 2.75 (m,1H,21-H or 22-H or 26-H or 27-H or 28-H), 3.13 (dd,13.4Hz,1.8Hz,1H,11-H), 3.62 (d,13.4Hz,1H,11-H), 5.45 (s,1H,5-H), 6.35 (s,1H,NH).13C-NMR (d-chloroform, 90.5 MHz):
21.3 (CH3,C-12 or C-13), 27.4 (CH,C-23 or C-24 or C-25), 27.4 (CH2,C-8 or C-9), 28.3 (CH3,C-13 or C-12), 28.4 (CH,C-17), 28.9 (CH,C-23 or C-24 or C-25), 30.8 (CH2,C-9 or C-8), 31.1 (CH,C-23 or C-24 or C-25), 36.8 (CH2,C-18), 37.3 (CH2,C-21 or C-22 or C-26 or C-27 or C-28), 38.8 (CH2,C-21 or C-22 or C-26 or C-27 or C-28), 39.2 (CH2,C-21 or C-22 or C-26 or C-27 or C-28), 39.9 (CH2,C-21 or C-22 or C-26 or C-27 or C-28), 40.2 (CH2,C-21 or C-22 or C-26 or C-27 or C-28), 40.9 (C,C-15 or C-19), 41.0 (CH2,C-16),43.8 (C,C-19 or C-15), 46.0 (CH,C-20), 47.0 (C,C-10), 53.6 (CH,C-14), 56.2 (CH2,C-11), 57.6 (C,C-1), 65.8 (CH,C-7), 115.2 (CH,C-5), 138.0 (C,C-3 or C-4), 151.5 (C,C-4 or C-3), 209.3 (C,C-6).
In the product C, it is very difficult to distinguish between the non-equivalent CH2 groups (and CH groups as well) of the adamantane residues. Further NMR work is in progress in order to get a complete assignment of all signals.