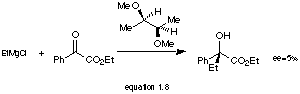
Asymmetric Grignard Chemistry
The reaction of a Grignard Reagent with an aldehyde or ketone results in a secondary or tertiary alcohol that has a chiral centre. The exclusive formation of the R or S isomer has long been a goal.[25] Current approaches to optically pure alcohols are by the following methods: (a) the reduction of ketones with chiral aluminium alkoxides, magnesium alkoxides or Grignard reagents (b) the reduction of ketones with chiral metal hydride reagents (c) the hydrogenation of ketones using a rhodium(I) complex as a catalyst.
Initial studies of enantioselective Grignard reagents used chiral ethers.[26] The stereoselectivity is generally poor (1-18%) as this typical example shows (equation 1.8):
Other approaches to asymmetric Grignard chemistry include the use of chiral substrates, ligands or catalysts. Chiral ligands and substrates containing heteroatoms are most desirable because of their ability to bind strongly to the metal centre and thus enforce enantiofacial selection. Chiral amines are the most used general class of ligands.
Mukaiyama et al[27] used a diamine 1.22 derived from (S)-Proline as a ligand for the asymmetric addition of an alkyl lithium to an aldehyde. Studies with other metal alkyls were carried out, and it was found that dialkylmagnesium was the most effective giving the highest chemical and optical yields (Table 1.1). The ligand is easily removed from the product by washing with aqueous HCl.

More recently, Tomioka and coworkers[28] have used the novel chiral amines 1.23 and 1.24 for the 1,2 addition of Grignard reagents to aldehydes. Optical yields were in the range of 36-75% with chemical yields as high as 96%.
Table 1.1. Chemical yield and e.e. for the reaction of metal alkyls with benzaldehyde in the presence of 1.22.
Metal Alkyl e.e. Chemical yield RCu 0 22 R2Zn 0 76 R3Al a RMgBr 47 90 R2Mg 68 93 a Benzyl alcohol obtained in 28% yield.
Optical purity increased with decreasing temperature but increasing the ratio of Grignard reagent and diamine to aldehyde had no significant effect on the enantioselectivity. Tomioka and coworkers[28] suggested that the monomeric structure 1.25 was probably the active species, with the steric interaction between the aryl group on the chiral ligand and R1 on the aldehyde being responsible for enantioface selection (Scheme 1.11).
Scheme 1.11. Enantiofacial selection for the reaction of a ketone with 1.25.
Normally, the diamine 1.26 gave e.e.'s of 86-97% in most cases for the 1,2-addition of a Grignard reagent to a ketone.[29] Interestingly, the addition of 1 equivalent of triethylamine led to the formation of the opposite product in similarly high e.e. (Scheme 1.12). The solvent is thought to be responsible for this highly desirable reversal in enantiofacial selection.
Scheme 1.12. Proposed effect of NEt3 on the enantioface selectivity of a ketone when 1.26 is coordinated to a Grignard reagent
Seebach and coworkers[30] reported excellent results using 1.27. Diol 1.27 was deprotonated with 2 equivalents of Grignard reagent before a further equivalent was added. Addition of ketone yielded the tertiary alcohol with yields of 60-90% with acetophenone and e.e.'s >98% (equation 1.11). Even higher e.e.'s may be possible with 1.27b because of its greater bulk, but even with 1.27a the enantioselectivity is unprecedented. The mechanism is not yet known, but is probably similar to that of the chiral diamine 1.26.
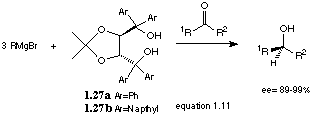
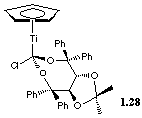
The TADDOL reagent 1.28 was the Fluka reagent of the year in 1995
for its unparalleled stereocontrol on the addition of allyl nucleophiles
to aldehydes.[31],[32] The ligand and the Ti can be recovered after the reaction
mixture has been hydrolysed.