C3 Symmetric Ligands
The tris(pyrazolyl)hydroborates 1.29 and 1.30 are two of a relatively few examples of optically active ligands that have C3 symmetry. It might appear paradoxical having a symmetry element within a chiral auxiliary whose function is to provide asymmetric induction in a chemical transformation. However, enantioface differentiation requires only that the auxiliary lacks mirror or inversion symmetry and therefore need not be symmetric only disymmetric. A wide range of C2- symmetric auxiliaries are used for asymmetric induction.[39]
High optical yields obtained with catalysts bearing C2 symmetric ligands have been attributed to a reduction in the number of possible diastereomeric transition states because of equivalent asymmetric environments imposed along the Z axis (+ and -) of an intermediate square planar metal complex. An octahedral intermediate bearing a chiral C2 symmetric ligand offers two non-equivalent coordination sites, axial and equatorial, whereas a similar octahedral intermediate with a chiral tridentate C3-symmetric ligand offers only one type of site for substrate coordination i.e. all sites are equivalent.
The reduced number of competing asymmetric environments has been proposed to lead to high enantioselectivity in catalytic reactions involving metal centres bearing chiral tripodal C3 symmetric ligands.[40]

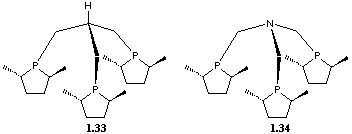
Examples of C3 symmetric ligands used in asymmetric induction are the tripodal phosphanes 1.33 and 1.34 developed by Burk et al.[40] Ligand 1.33 was reacted with [Rh(cod)2]+SbF6- to give the rhodium complex [Rh(cod)(1.33)]+SbF6- which is a catalytic precursor for the enantioselective hydrogenation of methyl (2)-a-acetoamidocinnamate (50oC, 89% e.e.) and dimethyl itaconate (50oC, 20h, 95% e.e.).
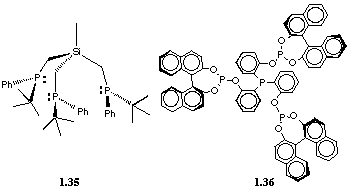
Other examples such as 1.35[41] or 1.36[42] have recently been made, but no catalytic properties have yet been explored.