 |
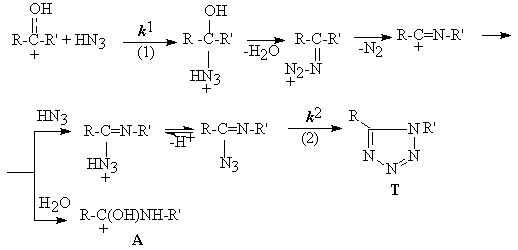
The Cycle Formation
3. 1,5-Disubstituted Tetrazoles by Schmidt Reaction
The rate constants for reaction of ketones with hidrogen azide in H2SO4 and for imidoyl azide cyclization (cycle formation) have been obtained in a wide range of medium acidity. The suitable kinetics equations (1,2) are solved in variable assosiated with medium acidity and basicity of reagents and intermediate.
 |
k1obs. = k1ho / (K1 + ho).(1+ho/K2+hoh+/K2K3); k2obs. = k2ho/K4+ho;
k1obs. , k1, k2obs., k2 - the observed and actual rate constants of ketone-HN3 interaction (1) and unprotonated imidoylazide cyclization (2); ho,h+ - the medium acidity for neutral (o) and charged (+) bases; K1,K2,K3,K4 - basicity constants for the ketone, HN3, H2N3+ and imidoyl azide respectively; F= cA/cT = K (fH2O cH2O/fHN3 cHN3 a ); K -kA/kT; fH2O, cH2O, fHN3 , cHN3 - activity coefficients and concentrates of water and hidrogen azide in H2SO4 .
1. G.I. Koldobskii, V.A.Ostrovskii, B.V.Gidaspov, Application of Schmidt reaction for synthesis of tetrazoles(Review), Khim.Geterotsikl.Soedin., # 6, 723-735 (1975).
2. A.S.Enin, G.I. Koldobskii, V.A.Ostrovskii, L.I.Bagal, Zh.Org.Khim., 8, # 9, 1895-1901 (1972).
3.V.A.Ostrovskii, A.S.Enin, G.I. Koldobskii, Zh.Org.Khim., 9, # 4, 802-806 (1973).
4. G.I. Koldobskii, A.S.Enin, V.N.Naumov, V.A.Ostrovskii, Zh.Org.Khim., 8, # 1, 242-245 (1972).