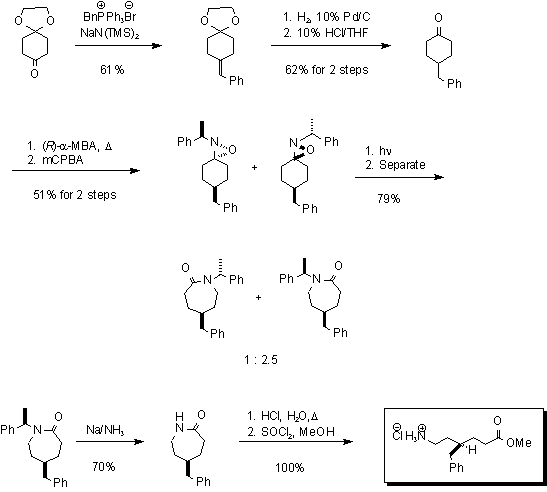
Synthesis of the 4-Benzyl-Aca Linker
The 4-benzyl-Aca linker is similarly derived from 4-benzylcyclohexanone.
In this example, an achiral ketone is the precursor in this stereoselective
synthesis of the desired lactam. The oxaziridine are formed as previously
described, however in this situation, the ring expansion yields a mixture
of lactams that, when subject to Birch conditions, eventually leads to
enantiomers.
Back to Index
Synthesis of Cyclic Peptides

