2.4. Lewis acid catalysis possible?
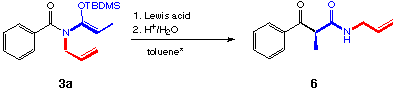
scheme 8
|
Nr. |
Lewis acid |
eq. catalyst |
T / °C |
T / h |
Yield (%) |
|
1 |
ZnCl2 |
0.7 |
85 |
22 |
85 |
|
2 |
ZnBr2 |
0.7 |
75 |
22 |
65 |
|
3 |
TiCl4 |
0.1 |
85 |
13 |
13 |
The (Cp)2ZnCl2, BF3*OEt2 and AlCl3 showed no catalytic effect.
No example has been reported where an aza-Claisen rearrangement starting from N,O-silylketene acetals has been catalyzed. For the aza-Cope rearrangements a large variety of Lewis-acid catalysts have been applied successfully [26-30]. It is proposed that the effect of the Lewis acids is the stabilization of the ionic intermediate, which is formed by initial bond breakage [31]. So we tried to catalyze the rearrangement of our substrate with Lewis acids under conditions which were already used by Stille (0.7 eq ZnCl2, toluene)[29].
Replacing the benzyl group by a benzoyl group increased the stability as mentioned before. At the same time the benzoyl group facilitated another side reaction: an intramolecular acylation. No rearrangement product was detected in that case. Cross-over experiments were undertaken to distinguish between the inter- and the intramolecular mechanism. Compounds 3b and 3d were heated with ZnCl2 in one pot. No cross-over products have been formed. Therefore we concluded that the reaction mechanism must be an intramolecular one.
![]()
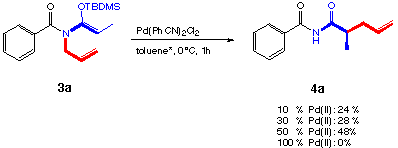
scheme 9
L. E. Overman showed in his review published in 1984 [32] that [3,3]-sigmatropic rearrangements can be catalyzed with Pd(II)- and Hg(II)-catalysts. He proposes that the "weak" elektrophiles Pd(II) catalyze allylimidate- and allylester-rearrangements much better than Lewis acids. He assumes that they induce a cyclisation which is responsible for the catalytic acceleration. Many papers have been published on this subject, e.g. [13,33-36]. Nowadays even methods for the enantioselective catalysis of the rearrangements have been reported [37,38].
Using Pd(II) we were able to catalyze the rearrangement of the adduct 3a. No starting material was left. But it was impossible to improve the yield of this reaction above 50 percent. We tried to add a strong ligand after the reaction to remove the palladium from our product without success. We supposed that the Pd(II) was forming a stable complex with the rearranged product. It was also impossible to improve the yield of the rearranged product by reduction of the palladium(II) in situ.
Catalysts like PdCl2, Pd(OAc)2, Pd2(allyl)2Cl2 and Hg(OCOCF3) showed no catalytic acceleration nor increased product formation.
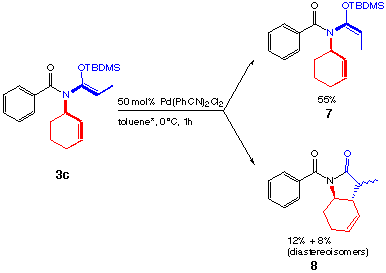
Different results are obtained if the allyl is replaced by a cyclohexenyl group (scheme 10):
scheme 10
In this case no rearranged product could be isolated. A possible mechanism for the formation of the product 7 could be the insertion and subsequent elimination of the Pd(II) into the double bond. Compounds like 8 are interesting, but unfortunately two diastereoisomers were formed in a ratio of 3 : 2. In view of the application of this rearrangement in our planned tandem reaction this side reaction was a serious problem.
![]()