
REGIOSELECTIVE IODINATION OF TOLUENE OVER ZEOLITES
Y
E.A. Zubkov, E.P. Muzhilov and V.G. Shubin
Institute of Organic Chemistry, Novosibirsk 630090, Russia
Iodoarenes are useful reagents in fine organic synthesis. Conventional methods for aromatic iodination imply the use of highly toxic heavy metal compounds (Hg, Tl etc.) or mineral acids [1] which is undesirable from the ecological point of view. Moreover, these methods do not always allow to control regioselectivity of the reaction. Zeolites are reputed to increase regioselectivity of electrophilic aromatic substitution reactions including halogenation [2]. It has been reported on the use of zeolites in iodination of aromatics [3-5]. However, there is a need in better understanding of the mechanism of iodination in the presence of zeolites. We have studied a liquid phase iodination reaction of toluene in the presence of several cation exchanged forms of zeolite Y (Table). High para-selectivity observed is obviously due to effect of shape selectivity. Electrophilic species formation was simulated by the model reaction (1) with the use of the MNDO method (MNDO-92 program [6]). Calculated heats of the reaction are given in the Table.

Table. Iodination of toluene in the presence of zeolites Y (20 oC, hexane, 24 hours) and calculated heats of reaction (1)
| Zeolite | Conversion of toluene, % | Calculated heat of reaction (1), kcal/mol |
| HY | 0 | - 0.1 |
| LiY | 0 | - 1.5 |
| NaY | 0 | -11 |
| KY | 28 a) | -29 |
| CsY | 64 a) | -40 |
a) para-Iodotoluene is the only product observed
These results seem to be in agreement with the Raman spectra of iodine adsorbed on zeolites Y (Figure).
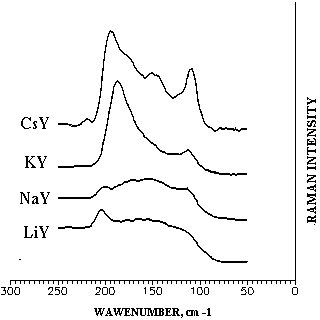
Figure. Raman spectra of iodine adsorbed on zeolites Y
The bands at 195-205 cm-1 may correspond to "physically adsorbed" iodine molecules (cf. a band at 210 cm-1 for the solution of iodine in hexane). When passing from LiY to CsY one can observe intensity increasing of the band at 110-120 cm-1 which probably corresponds to iodine molecules polarised by alkali metal cations inside zeolite lattice because "soft" iodine molecules are more effectively polarised by "softer" Cs and K cations than by "harder" Na and especially Li cations. Based on the reactivity and regioselectivity observed as well as on the quantum chemical simulations and Raman spectra of iodine adsorbed on zeolites we can presume that iodination reaction of toluene occurs inside zeolite cavities, electrophilic species being iodine molecules polarised by alkali metal cations.
References