

Next: 2. Experimental
Up: Chiral Silatranes
Previous: Chiral Silatranes
The search for organic materials for non-linear optics (NLO) has revealed
quite many compounds with high efficiency for frequency doubling of laser
light, but they suffer from a lack of transparency. This is due to the
generally accepted design of a NLO material which consists in connecting
a donor and an acceptor group by a conjugated bridge which leads to highly
coloured materials. To avoid the problems with this approach, we started
to develop compounds whose hyperpolarizability relies on the existence
of weak bonds. Here, the silatranes represent a well-studied class
of stable molecules with a weak Si-N bond.
Silatranes (2,8,9-trioxa-5-aza-1-silabicyclo[3.3.3]undecanes) are a special case
of pentacoordinate silicon with unusal properties which have gained
considerable interest over the last decades [1,2,3,4].
Interestingly, the silicon-nitrogen bond is ignored in the name used by
Chemical Abstracts (it should rather be a tricyclic system).
We introduced chirality as
only compounds which crystallize in non-centrosymmetric space groups give
non-vanishing hyperpolarizabilities as bulk materials, e.g. in crystalline
form, which is important for applications in devices. Only very few
silatranes with a chiral backbone are known up to now [5,6].
Starting with (1S)-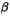 -pinene
as the source of chirality, we have prepared such a chiral silatrane and
characterized it by X-ray structure analysis [7].
-pinene
as the source of chirality, we have prepared such a chiral silatrane and
characterized it by X-ray structure analysis [7].
Figure 1:
X-Ray structure of our chiral silatrane
 |
|
If your browser is JavaApplet enabled you can drag your mouse below to rotate the molecule.
|
|
|


Next: 2. Experimental
Up: Chiral Silatranes
Previous: Chiral Silatranes
Bjoern Pedersen
1998-06-18
![]() -pinene
as the source of chirality, we have prepared such a chiral silatrane and
characterized it by X-ray structure analysis [7].
-pinene
as the source of chirality, we have prepared such a chiral silatrane and
characterized it by X-ray structure analysis [7].
