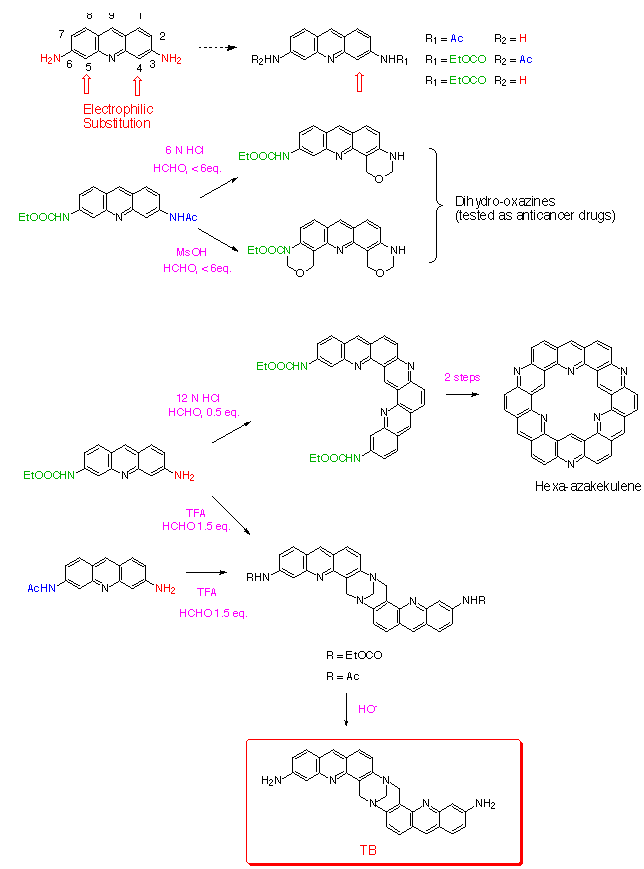
We recently reported that 3-aminoacridine
derivatives react with formaldehyde in acidic media [Tatibouët,
A.; Fixler, N.; Demeunynck, M.; Lhomme, J. Tetrahedron
1997, 53, 2891-2898] Along with the Tröger's bases,
the reaction may lead to a number of products including dihydrooxazine
and tetrahydroquinazoline derivatives and new heptacyclic heterocycles
[Tatibouët, A.; Hancock, R.; Demeunynck, M.; Lhomme, J.
Angew. Chem, Int. Ed. Engl. 1997, 36,1190-1191].
The product distribution depends on the nature of the acid used and
on the amine/formaldehyde ratio [Salez, H.; Wardani, A.; Tatibouet, A.;
Demeunynck, M.; Lhomme, J. Tetrahedron Lett. 1995,
36, 1271-1274]. The best yields in Tröger's Base were obtained
using 1.5 equivalents of formaldehyde and trifluoroacetic acid as solvent.
Synthesis of the Tröger's Base starting
from 3,6-diaminoacridine required protection of one of the amino
groups prior to reaction with formaldehyde to avoid side reactions.
The acetamido group introduced at position 3 proved convenient as it
is stable in trifluoroacetic acid and deactivates substitution at
carbon C-4 leading to selective reaction at C-5 ortho to the free
amino function. The heterocyclic Tröger's Base analogue was
prepared in two steps starting from monoacetylated proflavine.
Condensation of monoacetylproflavine with formaldehyde
in trifluoroacetic acid gave the diacetamido Tröger's Base
analogue in 76 % yield. Treatment in basic
conditions (EtOH, NaOH) allowed cleavage of the two acetamido
groups without degradation of the methano diazocine bridge.
The new Tröger's Base analogue was obtained in 90 % yield.