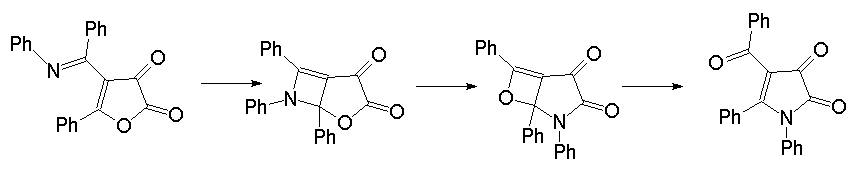
Rearrangement by an electrocyclic ring closure - ring opening sequence
The main features of this mechanistic possibility consists in an (hetero)electrocyclic
ring closure of the iminobenzyl group and the endocyclic double bond
in 1 to form a bicyclic product
which then might rearrange an isomeric bicyclus.
Subsequent (hetero)electrocyclic ring opening then could yield the pyrrole-2,3-dione
2.
The semi-empirical calculations reveal a rather complex reaction sequence
for this mechanism (energetic as well as structural details are provided
in Table 1). The calculated activation energies
for this reaction sequence are rather high - considerably higher than
those for elimination of carbon monoxide to give a-oxo
ketenes !
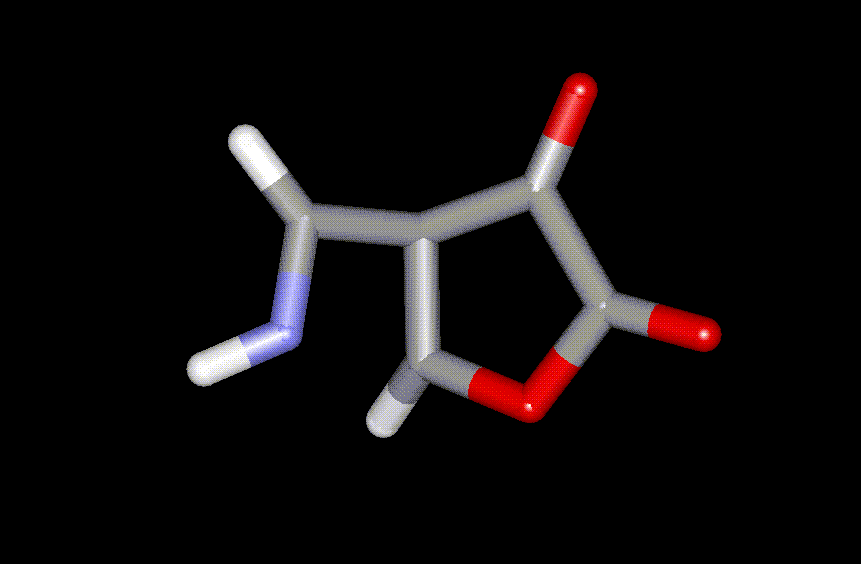
Figure 2. Transition state for electrocyclic ring
closure of 1 (rate determining step)
You have to install the ChemScape
CHIME plugin with Netscape 3.0 (or higher) in order to see the embedded
images and to manipulate the displayed molecules "live" on your screen
Alternatively you can download freeware programs such as RASMOL
or WEBLAB VIEWER that can read
the *.pdb file.
Formation of compound 2 by this mechanism,
therefore, would require prohibetively large activation energies and, thus,
is highly unlikely.

