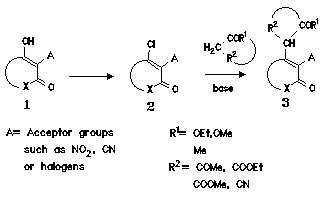
Another aspect of our work was the synthesis of heterocyclic substituted malonates, cyanoacetates or acetoacetates, which can be used as synthons for further cyclization reactions.
To study a general entry in this class of compounds, we started from 1-hydroxy-3-oxo compounds 1, which were converted to 1-chloro-3-oxo carbo- and heterocycles 2. By reaction with active methylene compounds such as malonates or acetoacetates the formation of 1-alkyl-3-oxo-carbo-and heterocycles 3 should be achieved. However, the yields of 3 depend strongly on the nature of the substituent A in position 2 of the former 1,3-dicarbonyl compound. We found that only electron withdrawing groups such as nitro- or cyano groups (and in a few cases halogen substituents) facilitated the alkylation to give in good yields the 1-alkyl-3-oxo-carbo-and heterocycles 3.

By this method we were able to synthesize a great variety of compounds by variation of the basic
ring system and the active methylene compound (a small seelction is shown in the
formula scheme below,
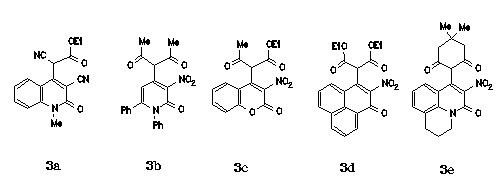
e.g. 2-quinolones and cyanoacetates in 3a, 2-pyridones and acetylacetone in 3b,
coumarins and acetoacetates in 3c, phenalenones and malonates in 3d or
benzoquinolizinones and dimedone in 3e.).
Synthesis of diethyl 2-(3-substituted-2-oxo-1,2-dihydroquinolin-4-yl)-malonates (compound of type 3)
A mixture of 4-chloro-3-substituted-2-quinolone (10 mmol), diethyl malonate (20 mmol) and anhydrous potassium carbonate was stirred at ambient temperature for 2-6 hours in dimethyl formamide. After dilution with ice/water the solution was slowly acidified with conc. hydrochloric acid to pH=1 to precipitate the product. After standing for some hours the precipitate was filtered and washed with water. Yield: 80-95%.
[2] P. D. Leeson, R. W. Carling, K. W. Moore, A. M. Moseley, J. D. Smith, G. Stevenson, T. Chan, R. Baker, A C. Foster, S. Grimwood, J. A. Kemp, G. R. Marshall, K. Hoogsten, J. Med. Chem. 35 (1992) 1954-68.
[3] Z. Y. Jiang, Q.-L. Zhou, J. W. Eaton, W. H. Koppenol, J. V. Hunt, S. P. Wolff, Biochem. Pharmacol. 42, (1991) 1273.
[4] M. Uchida, F. Tabusa, M. Komatsu, S. Morita, T. Kanbe, K. Nakagawa, Chem. Pharm. Bull. 33 (1985) 3775-86.