 Stereocontrolled
Nucleophilic Additions of Lithiated Heterocycles to Nitrones. Synthesis
and Structural Characterization of Novel Chiral (Hydroxyamino)methyl Heterocycles
Stereocontrolled
Nucleophilic Additions of Lithiated Heterocycles to Nitrones. Synthesis
and Structural Characterization of Novel Chiral (Hydroxyamino)methyl HeterocyclesPhysical and spectroscopic properties of (hydroxyamino)methylheterocycles
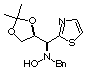 Compound
9a
Compound
9a
[a]D20 = -7.8
(c 0.74, CHCl3); oil
1H NMR (CDCl3) d 1.25
(s, 3H), 1.28 (s, 3H), 3.70 (dd, 1H, J = 5.7, 7.9 Hz), 3.84 (d, 1H, J =
12.0 Hz), 3.94 (dd, 1H, J = 5.7, 8.2 Hz), 3.98 (d, 1H, J = 12.0 Hz), 4.38
(d, 1H, J = 6.8 Hz), 4.72 (ddd, 1H, J = 6.8, 7.9, 8.2 Hz), 6.45 (bs, 1H,
ex. D2O), 7.20-7.35 (m, 5H), 7.38 (d, 1H, J = 3.2 Hz), 7.82
(d, 1H, J = 3.2 Hz).
13C NMR (CDCl3) d
25.5, 26.5, 61.7, 67.0, 68.6, 76.3, 109.6, 120.3, 127.5, 128.4, 129.4,
136.8, 142.0, 164.7.
Compound 9b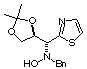
[a]D20 = -9.0
(c 0.39, CHCl3); mp 157-159°C
1H NMR (CDCl3) d 1.28
(s, 3H), 1.32 (s, 3H), 3.71 (d, 1H, J = 13.0 Hz), 3.79 (d, 1H, J = 13.2
Hz), 4.05 (dd, 1H, J = 5.3, 8.5 Hz), 4.15 (dd, 1H, J = 5.5, 8.5 Hz), 4.16
(d, 1H, J = 7.7 Hz), 4.72 (pseudo dt, 1H, J = 5.4, 7.7 Hz), 6.43 (bs, 1H,
ex. D2O), 7.24-7.32(m, 5H), 7.39 (d, 1H, J = 3.2 Hz), 7.82 (d,
1H, J = 3.2 Hz).
13C NMR (CDCl3) d
25.21, 26.7, 62.1, 67.7, 69.0, 76.5, 109.8, 120.1, 127.5, 128.3, 129.2,
136.9, 141.7, 165.5.
Compound 10a 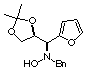
[a]D20 = -28.9
(c 0.79, CHCl3); oil
1H NMR (CDCl3) d 1.31
(s, 3H), 1.37 (s, 3H), 3.54 (dd, 1H, J = 5.8, 8.6 Hz), 3.70 (d, 1H, J =
12.9 Hz), 3.84-3.92 (m, 3H), 4.73 (pseduo dt, 1H, J = 6.3, 8.7 Hz), 6.10
(bs, 1H, ex. D2O), 6.34 (d, 1H, J = 3.2 Hz), 6.39 (dd, 1H, J
= 2.0, 3.2 Hz), 7.22-7.38 (m, 5H), 7.43 (d, 1H, J = 2.0 Hz).
13C NMR (CDCl3) d
25.5, 26.7, 61.7, 65.4, 67.1, 75.2, 109.8, 110.3, 110.4, 127.4, 128.3,
129.6, 137.0, 142.4, 149.9.
Compound 10b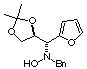
[a]D20 = -13.2
(c 1.00, CHCl3); mp 119-120°C
1H NMR (CDCl3) d 1.32
(s, 3H), 1.33 (s, 3H), 3.66 (d, 1H, J = 13.1 Hz), 3.72 (d, 1H, J
= 13.1 Hz), 3.87 (d, 1H, J = 8.1 Hz), 4.02 (dd, 1H, J = 5.9, 8.5 Hz), 4.14
(dd, 1H, J = 6.1, 8.5 Hz), 4.67 (ddd, 1H, J = 5.9, 6.1, 8.1 Hz), 4.75 (bs,
1H, ex. D2O), 6.43 (s, 2H), 7.22-7.38 (m, 5H), 7.48 (d, 1H,
J = 1.1 Hz).
13C NMR (CDCl3) d
25.5, 26.7, 62.1, 66.1, 67.9, 75.6, 109.5, 110.4, 110.5, 127.5, 128.4,
129.4, 137.2, 142.4, 150.1.
Compound 11a 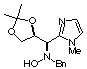
[a]D20 = + 17.2
(c 1.50, CHCl3); mp 103-105°C
1H NMR (CDCl3) d 1.31
(s, 3H), 1.32 (s, 3H), 3.42 (s, 3H), 3.57 (d, 1H, J = 12.3 Hz), 3.90 (dd,
1H, J = 7.8, 9.0 Hz), 4.18 (dd, 1H, J = 6.3, 9.0 Hz), 4.21 (d, 1H, J =
12.3 Hz), 4.24 (d, 1H, J = 5.0 Hz), 4.69 (ddd, 1H, J = 5.0, 6.3, 7.8 Hz),
6.84 (d, 1H, J = 1.7 Hz), 7.02 (d, 1H, J = 1.7 Hz), 7.15 (bs, 1H, ex. D2O),
7.24-7.33 (m, 5H).
13C NMR (CDCl3) d
24.6, 26.3, 32.7, 61.4, 62.2, 68.2, 76.9, 109.0, 120.8, 126.7, 127.5, 128.4,
128.8, 136.5, 145.8.
Compound 11b 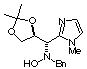
[a]D20 = -14.2
(c 0.88, CHCl3); mp 118-120°C
1H NMR (CDCl3) d 1.24
(s, 3H), 1.30 (s, 3H), 3.38 (s, 3H), 3.42 (d, 1H, J = 12.4 Hz), 3.57 (d,
1H, J = 9.3 Hz), 4.05 (dd, 1H, J = 5.7, 8.9 Hz), 4.09 (d, 1H, J = 12.4
Hz), 4.30 (dd, 1H, J = 6.5, 8.9 Hz), 4.75 (ddd, 1H, J = 5.8, 6.4, 9.4 Hz),
6.84 (d, 1H, J = 1.7 Hz), 7.04 (d, 1H, J = 1.7 Hz), 7.12 (bs, 1H, ex. D2O),
7.30-7.36 (m, 5H).
13C NMR (CDCl3) d
25.5, 25.6, 32.8, 59.7, 62.1, 66.7, 77.3, 108.1, 120.7, 126.8, 127.4, 128.3,
129.0, 136.7, 143.8.
Compound 12a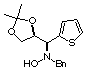
Rf (hexano:diethyl ether, 60:40) = 0.55; [a]D20
= -24.9 (c 0.41, CHCl3); mp 117-119°C
1H NMR (CDCl3) d 1.37
(s, 3H), 1.42 (s, 3H), 3.48 (dd, 1H, J = 5.5, 9.0 Hz), 3.65 (d, 1H, J =
12.7 Hz), 3.75 (dd, 1H, J = 6.5, 9.0 Hz), 3.96 (d, 1H, J = 12.7 Hz), 4.00
(d, 1H, J = 9.4 Hz), 4.72 (ddd, 1H, J = 5.5, 6.5, 9.4 Hz), 5.35 (bs, 1H,
ex. D2O), 6.9-7.4 (m, 8H).
13C NMR (CDCl3) d
225.6, 26.8, 61.4, 67.3, 72.1, 76.6, 110.1, 121.9, 123.8, 126.1, 126.6,
127.4, 128.5, 135.8, 137.0.
Compound 12b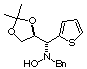
Rf (hexano:diethyl ether, 60:40) = 0.65; [a]D20
= -14.6 (c 0.32, CHCl3); mp 126-128°C
1H NMR (CDCl3) d 1.30
(s, 3H), 1.35 (s, 3H), 3.60 (d, 1H, J = 13.3 Hz), 3.78 (d, 1H, J = 13.3
Hz), 3.97 (dd, 1H, J = 6.3, 8.3 Hz), 4.05 (d, 1H, J = 7.2 Hz), 4.11 (dd,
1H, J = 6.5, 8.3 Hz), 4.65 (ddd, 1H, J = 6.3, 6.5, 8.3 Hz), 4.74 (bs, 1H,
ex. D2O), 6.9-7.6 (m, 8H).
13C NMR (CDCl3) d
25.4, 26.7, 61.8, 67.3, 68.3, 77.0, 109.6, 122.1, 126.2, 126.4, 127.4,
128.3, 129.4, 136.8, 137.3.
Compound 13a 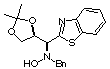
Rf (hexano:diethyl ether, 60:40) = 0.48; [a]D20
= -25.7 (c 0.36, CHCl3); mp 153-155°C
1H NMR (CDCl3) d 1.37
(s, 3H), 1.39 (s, 3H), 3.85 (dd, 1H, J = 6.5, 9.0 Hz), 4.00 (dd, 1H, J
= 6.7, 9.0 Hz), 4.05 (d, 1H, J = 13.0 Hz), 4.12 (d, 1H, J = 13.0 Hz), 4.47
(d, 1H, J = 6.6 Hz), 4.83 (pseduo q, 1H, J = 6.5 Hz), 6.06 (bs, 1H, ex.
D2O), 7.20-7.42 (m, 7 H), 7.91 (d, 1H, J = 80 Hz), 8.03 (d,
1H, J = 8.0 Hz).
13C NMR (CDCl3) d
25.6, 26.7, 62.0, 67.3, 70.0, 76.2, 109.8, 119.8, 121.7, 123.4, 125.6,
126.2, 127.7, 128.5, 129.4, 135.3, 137.1, 170.7.
Compound 13b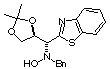
Rf (hexano:diethyl ether, 60:40) = 0.37; [a]D20
= -31.2 (c 0.40, CHCl3); mp 188-190°C
1H NMR (CDCl3) d 1.29
(s, 3H), 1.37 (s, 3H), 3.83 (d, 1H, J = 13.0 Hz), 3.95 (d, 1H, J = 13.0
Hz), 4.16 (dd, 1H, J = 5.1, 8.8 Hz), 4.19 (d, 1H, J = 8.5 Hz), 4.22 (dd,
1H, J =6.1, 8.8 Hz), 4.81 (ddd, 1H, J = 5.1, 6.1, 8.5 Hz), 6.36 (bs, 1H,
ex. D2O), 7.24-7.53 (m, 7H), 7.92 (d, 1H, J = 8.0 Hz), 8.07
(d, 1H, J = 8.0 Hz).
13C NMR (CDCl3) d
225.6, 27.1, 62.5, 68.0, 70.3, 77.0, 109.9, 119.4, 121.7, 123.3, 125.5,
126.2, 127.8, 128.6, 129.3, 136.0, 136.8, 170.4.
Compound 14a 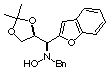
Rf (hexano:diethyl ether, 60:40) = 0.35; [a]D20
= -30.4 (c 0.26 , CHCl3); mp 129-131°C
1H NMR (CDCl3) d 1.38
(s, 3H), 1.42 (s, 3H), 3.67 (dd, 1H, J = 6.0, 8.7 Hz), 3.82 (d, 1H, J =
13.0 Hz), 3.95 (dd, 1H, J = 6.8, 8.7 Hz), 3.98 (d, 1H, J = 13.0 Hz), 4.07
(d, 1H, J =8.5 Hz), 4.84 (pseudo dt, 1H, J = 6.2, 8.5 Hz), 5.80 (bs (1H,
ex. D2O), 6.76 (s, 1H), 7.20-7.464 (m, 9H).
13C NMR (CDCl3) d
25.6, 26.7, 61.8, 65.9, 67.1, 75.1, 111.3, 121.1, 122.9, 124.2, 124.3,
127.4, 128.1, 128.3, 128.4, 129.4, 129.7, 137.0, 152.7.
Compound 14b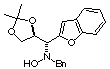
Rf (hexano:diethyl ether, 60:40) = 0.49; [a]D20
= -22.3 (c 0.41, CHCl3); mp 97-99°C
1H NMR (CDCl3) d 1.32
(s, 3H), 1.34 (s, 3H), 3.81 (d, 1H, J =13.0 Hz), 3.82 (d, 1H, J = 13.0
Hz), 4.01 (d, 1H, J = 8.6 Hz), 4.12 (dd, 1H, J =5.7, 8.7 Hz), 4.20 (dd,
1H, J = 6.1, 8.7 Hz), 4.60 (bs, 1H, ex. D2O), 4.77 (pseudo dt,
1H, J = 5.8, 8.6 Hz), 6.82 (s, 1H), 7.15-7.43 (m, 9H).
13C NMR (CDCl3) d
25.5, 26.8, 61.8, 66.2, 67.2, 75.2, 111.4, 121.2, 122.8, 124.1, 127.5,
127.6, 128.3, 128.4, 129.4, 137.1, 153.1.
Compound 15a 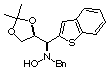
Rf (hexano:diethyl ether, 60:40) = 0.50; [a]D20
= -35.2 (c 0.30 , CHCl3); mp 102-104°C
1H NMR (CDCl3) d 1.38
(s, 3H), 1.40 (s, 3H), 3.60 (dd, 1H, J = 5.7, 8.8 Hz), 3.76 (d, 1H, J =
13.0 Hz), 3.85 (dd, 1H, J =6.4, 8.8 Hz), 3.99 (d, 1H, J = 13.0 Hz), 4.08
(d, 1H, J = 8.9 Hz), 4.77 (pseudo dt, 1H, J = 5.8, 8.9 Hz), 6.53 (bs, 1H,
ex. D2O), 7.17 (s, 1H), 7.20-7.40 (m, 9H).
13C NMR (CDCl3) d
25.6, 26.9, 61.6, 67.2, 68.2, 76.6, 110.1, 122.2, 123.4, 124.2, 124.5,
125.1, 127.4, 128.3, 129.5, 137.0, 137.5, 138.7, 140.8.
Compound 15b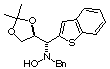
Rf (hexano:diethyl ether, 60:40) = 0.39; [a]D20
= -22.3 (c 0.60, CHCl3); mp 127-129°C
1H NMR (CDCl3) d 1.33
(s, 3H), 1.39 (s, 3H), 3.70 (d, 1H, J =13.2 Hz), 3.86 (d, 1H, J = 13.2
Hz), 4.09 (dd, 1H, J =5.9, 8.6 Hz), 4.13 (d, 1H, J = 7.6 Hz), 4.19 (dd,
1H, J = 6.2, 8.6 Hz), 4.72 (ddd, 1H, J = 5.9, 6.2, 7.6 Hz), 4.85 (bs, 1H,
ex. D2O), 7.18 (s, 1H), 7.21-7.46 (m, 9H)..
13C NMR (CDCl3) d
25.3, 26.7, 62.0, 68.0, 76.6, 109.6, 122.2, 124.5, 124.1, 124.2, 125.1,
127.5, 128.4, 129.4, 137.2, 137.3, 138.6, 139.0.