D01 DBFOX/Ph Complex Catalyzed Nitrone Cycloadditions
Catalyzed Asymmetric Nitrone Cycloadditions:
1,3-Dipolar cycloadditions to alkene dipolarophiles
are now the most useful method to make stereochemically defined five-membered
heterocycles. Although a variety of diastereoselective 1,3-dipolar cycloadditions
have been developed, enantioselective versions are still limited. Nitrones
are important 1,3-dipoles that have been the target of catalyzed enantioselective
reactions. Three different approaches to catalyzed enantioselective reactions
have been taken:
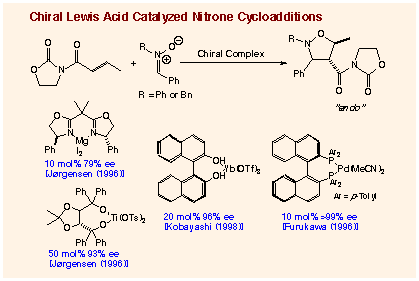
(1) activation of electron
deficient alkenes by a chiral Lewis acid,
(2)
activation of nitrones in the reaction with ketene acetals,
(3) coordination of both nitrones and allylic
alcohols on a chiral catalyst.
Among
these approaches, the dipole/HOMO controlled reactions of electron deficient
alkenes such as N-alkenoyl-2-oxazolidinones or N-alkenoylsuccinimides must
be most promising because a variety of combinations between chiral Lewis
acids and electron deficient alkenes have been well investigated in the
study of catalyzed enantioselective Diels-Alder reactions. Enantioselectivities
in catalyzed nitrone cycloadditions sometimes exceed 90% ee, but the efficiency
of catalytic loading remains insufficient.

DBFOX/Ph - Transition Metal Complexes as Catalyst:
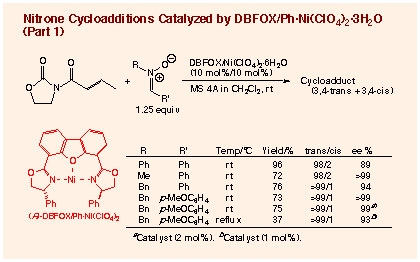
Among the transition metal - DBFOX/Ph complexes
examined, the best catalyst was the aqua complex of DBFOX/Ph ligand with
nickel(II) perchlorate. This catalyst is easy to prepare from commercially
available and inexpensive nickel perchlorate hexahydrate, and can be stored
for months in open air without loss of catalytic activity. In the presence
of 10 mol% of the anhydrous nickel catalyst, which can be prepared in situ
from R,R-DBFOX/Ph ligand, NiBr2, and 2 equimolar amounts of AgClO4, the
reaction of 3-crotonoyl-2-oxazolidinone with N-benzylidene-methylamine N-oxide
produces 3,4-trans-isoxazolidine in the perfect endo-selectivity (endo:exo
= 99:1) and enantioselectivity for the 3R,4S,5R-enantiomer (>99% ee for
endo-isomer). The aqua nickel complex, which can be simply prepared in situ
by stirring equimolar amounts of the R,R-DBFOX/Ph ligand and Ni(ClO4)2´6H2O
in dichloroethane for a few hours, gives a comparable result in a similar
reaction in the presence of MS 4A. The simple preparation procedure of the
aqua catalyst should be attractive.
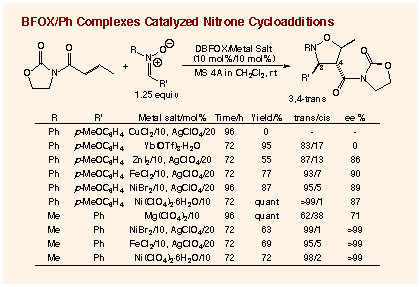
Effective Nitrone Cycloadditions Catalyzed DBFOX/Ph´Ni(ClO4)2:
Among the transition metal - DBFOX/Ph complexes
examined, the best catalyst was the aqua complex of DBFOX/Ph ligand with
nickel(II) perchlorate. This catalyst is easy to prepare from commercially
available and inexpensive nickel perchlorate hexahydrate, and can be stored
for months in open air without loss of catalytic activity. In the presence
of 10 mol% of the anhydrous nickel catalyst, which can be prepared in situ
from R,R-DBFOX/Ph ligand, NiBr2, and 2 equimolar amounts of AgClO4, the
reaction of 3-crotonoyl-2-oxazolidinone with N-benzylidene-methylamine N-oxide
produces 3,4-trans-isoxazolidine in the perfect endo-selectivity (endo:exo
= 99:1) and enantioselectivity for the 3R,4S,5R-enantiomer (>99% ee for
endo-isomer). The aqua nickel complex, which can be simply prepared in situ
by stirring equimolar amounts of the R,R-DBFOX/Ph ligand and Ni(ClO4)2´6H2O
in dichloroethane for a few hours, gives a comparable result in a similar
reaction in the presence of MS 4A. The simple preparation procedure of the
aqua catalyst should be attractive.
Presence of MS 4A is essential to attain high selectivities, especially in the reactions catalyzed by the aqua complex. In the absence of MS 4A, endo-selectivity and enantioselectivity for 3,4-trans-isoxazolidines are both lowered. Jorgensen was the first to observe a dramatic effect of MS 4A in Lewis acid catalyzed nitrone cycloadditions. In his reaction, the chemical yield of the cycloadduct was not affected by the absence of MS 4A, but the endo-selectivity was lowered (endo:exo: from 92:8 to 65:35) and the enantioselectivity almost disappered (79 to 2% ee). Our result is comparable. Although the role of MS 4A cannot yet be fully explained, it certainly works as dehydrating agent. In a reaction catalyzed by the aqua nickel complex, anhydrous magnesium sulfate can replace MS 4A, while the reaction becomes a little slower.
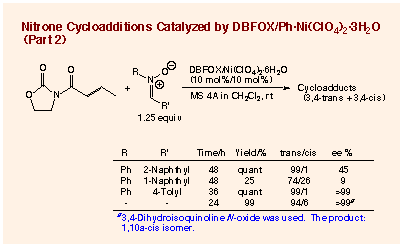
Reactions of other nitrones are also diastereoselective (endo:exo >95:5) and enantioselective for endo-isoxazolidines (99 - 89% ee). High efficiency of the catalytic cycle can be demonstrated in the reactions of N-(p-methoxybenzylidene)benzylamine N-oxide: a 99% ee for the endo-cycloadduct is recorded with 2 mol% of the catalyst at room temperature and a 93% ee with 1 mol% even under reflux in dichloromethane. The nitrone having a bulky aromatic C-substituent such as N-(1-naphthylidene)aniline N-oxide shows a rather decreased reactivity, the chemical yield, diastereoselectivity, and enantioselectivity being all poor, while the reaction with N-(2-naphthylidene)aniline N-oxide is sufficiently reactive. It is pleasing that N-propylidenebenzylamine N-oxide derived from an aliphatic aldehyde shows excellent diastereoselectivity as well as enantioselectivity for the endo-cycloadduct. Thus, DBFOX/Ph´Ni(ClO4)2´3H2O catalyzed asymmetric nitrone cycloaddition in the presence of MS 4A has the most promising featurest with respect to the catalytic cycle, diastereoselectivity, and enantioselectivity among the catalyzed reactions yet reported.