B02 Preparation of DBFOX/Ph Complexes and Structures of Active Catalysts
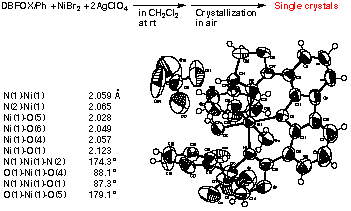
Structure of DBFOX/Ph“Ni(ClO4)2“3H2O (Real Catalyst):
Although ligand R,R-DBFOX/Ph is difficult to crystallize,
the crystallization of the nickel complex is relatively easy. Thus, the
anhydrous complex DBFOX/Ph“Ni(ClO4)2 was crystallized from acetone - dichloromethane
to give fine crystals. The X-ray stereostructure indicates that the complex
has a molecular formula of DBFOX/Ph“Ni(ClO4)2“3H2O with an octahedral structure.
The two oxazoline rings are coplanar with the plane of bibenzofuran, forming
a beautiful C2-symmetric chiral structure. The nickel ion is bound to three
water molecules and the furan oxygen atom. The N-Ni-N bond is almost linear
(<N-Ni-N = 174.2Į), indicating that DBFOX/Ph 1 is trans-chelating toward
Ni(II) ion.31 The two N-Ni distances are 2.059 and 2.065 !which are typical
of bond distances between an imine nitrogen and a nickel ion. To our great
delight, the isolated crystal was found to be an active catalyst in the
Diels-Alder reaction leading to the absolute enantioselectivity for endo-cycloadduct
at -40 ĮC.

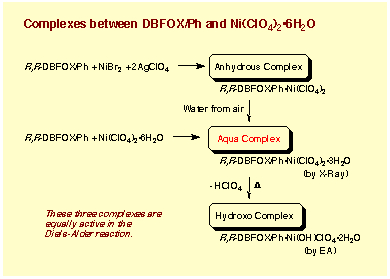
Anhydrous, Trihydrate, and Hydroxo Complexes:
This catalyst shows high water tolerance against
moisture in air. Accordingly, we prepared the aqua complex directly from
DBFOX/Ph ligand R,R-DBFOX/Ph and Ni(ClO4)2“6H2O. Thus, a pale blue solid
can be isolated when Ni(ClO4)2“6H2O is treated with R,R-DBFOX/Ph in dichloromethane,
followed by evaporation of the solvent. This solid shows a high catalytic
activity and enantioselectivity in the Diels-Alder reaction (-40 ĮC, quant,
endo:exo = 97:3, >99% ee), and these attractive characteristics remained
even after storage in air at room temperature for three months . Although
this complex shows an almost the identical IR spectrum to that of the above
crystal (DBFOX/Ph“Ni(ClO4)2“3H2O), it has a hydroxo monoperchlorate complex
structure on the basis of elemental analysis. The molecular formula, DBFOX/Ph“Ni(ClO4)(OH)“2H2O,
corresponds to a product formed by elimination of a perchloric acid from
DBFOX/Ph“Ni(ClO4)2“3H2O. We believe that the real active catalyst in reaction
is diperchlorate trihydrate (DBFOX/Ph“Ni(ClO4)2“3H2O), and that loss of
perchloric acid leading to A occurred during the drying procedure. However,
hydroxo monoperchlorate complex has also a strong catalytic activity in
the Diels-Alder reaction to show the absolute enantioselectivity (-40 ĮC,
48 h, 99%, endo:exo = 97:3, >99% ee).