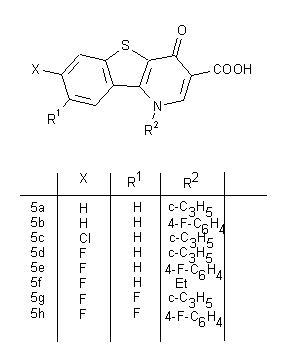
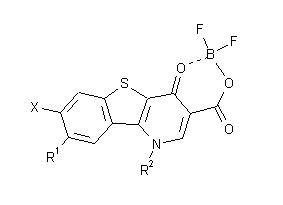
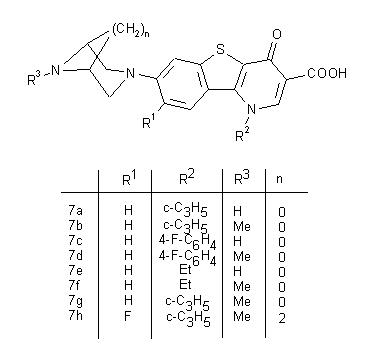
| Keywords |
Quinolones / Gyrase Inhibitors / lin-benzo / Grohe quinolone synthesis / electronic publishing / |
| ECHET96 Electronic Publishing Note |
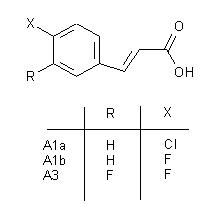
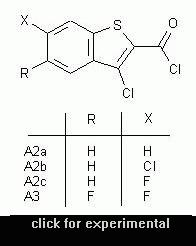
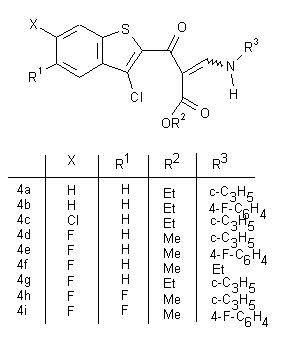
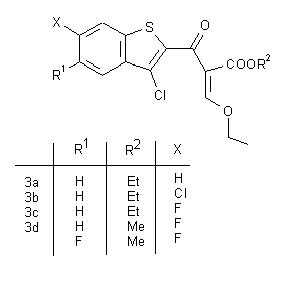
This poster consists of a clickable table of the reaction scheme.
Clicking on the structures of the scheme leads to a link of a scanned image of the corresponding experimental part of the thesis of Peter Martinek.
Details of the process and software used to produce this paper are described here
|
| Abstract |
The synthesis of the title compounds A focusing on the regioselective nucleophilic aromatic substitution
of 1-substituted 7,8-difluoro-1,4-dihydro-4-oxo- [1]benzothieno[3,2-b]pyridine-3-carboxylic anhydrides with difluoroboric acid (B) as potential antibacterials is described:
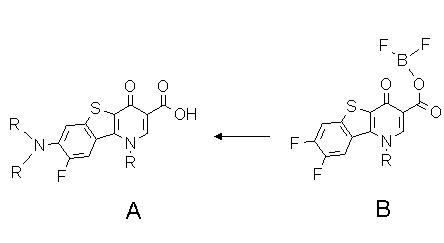 |
|
See also the accompanying poster "Who can model new quinolone-type heterocycles as potential
antibacterials? Synthesis of thieno[2',3':4,5]thieno[3,2-b]pyridone-3-carboxylic acids" |