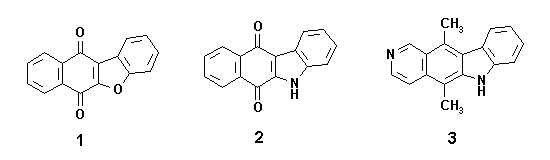

Ketoacids 4 have been shown to be interesting starting materials for the single and efficient synthesis of benzofuranonaphthoquinones 1 and indolonaphthoquinones 2, because they have appropriate carbon skeletons and funtionality for such transformations.
In fact, we have previously transformed bromoketoesters 4 (X=Br; R=H, OMe) into benzofuranonaphthoquinones 1 (R=H, OMe) and nitroketoesters 4 (X=NO2; R=H, OMe) into indolonaphthoquinones 2 (R=H, OMe), respectively.
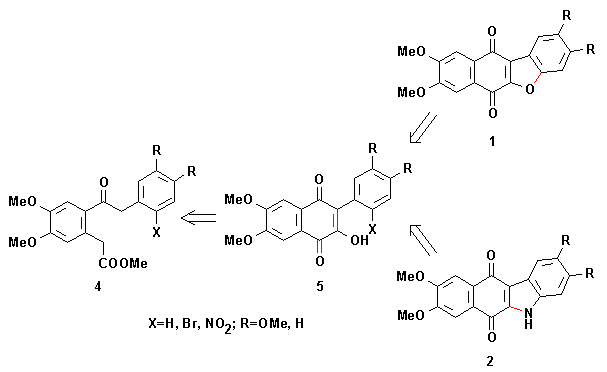
Treatment of bromoketoesters 4 (X=Br; R=H, OMe) with sodium hydroxide yielded bromoquinones 5 (X=Br; R=H, OMe), probably by mixed Claisen condensation followed by oxidation of the cyclized product. An intramolecular Ullmann coupling reaction of these bromoquinones 5 gave benzofuranonaphthoquinones 1 (R=H, OMe).
Nitroquinones 5 (X=NO2; R=H, OMe) can be obtained in a similar way from nitroketoesters 4 (X=NO2; R=H, OMe). Reduction of nitroquinones 5 to its amino derivatives and subsequent conjugate addition of NH2 to the quinone ring leads to indolonaphthoquinones 2 (R=H, OMe).
The necessary ketoacids 4 were prepared by Friedel-Crafts type acylations, this not being a general way to these compounds.
Therefore, this synthesis of benzofuranonaphthoquinones and indolonaphthoquinones requires the investigation of general methods for the preparation of phenylacetylphenylacetic acids 4.

